PDF - Neural Development
PDF - Neural Development
PDF - Neural Development
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Kizil et al. <strong>Neural</strong> <strong>Development</strong> 2012, 7:27 Page 9 of 13<br />
http://www.neuraldevelopment.com/content/7/1/27<br />
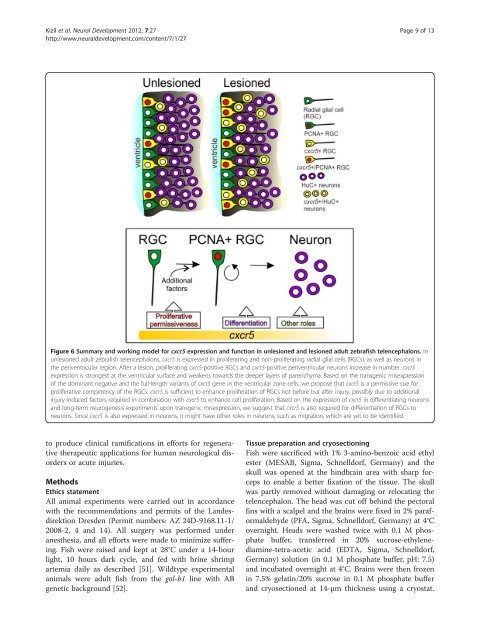
Figure 6 Summary and working model for cxcr5 expression and function in unlesioned and lesioned adult zebrafish telencephalons. In<br />
unlesioned adult zebrafish telencephalons, cxcr5 is expressed in proliferating and non-proliferating radial glial cells (RGCs), as well as neurons in<br />
the periventricular region. After a lesion, proliferating cxcr5-positive RGCs and cxcr5-positive periventricular neurons increase in number. cxcr5<br />
expression is strongest at the ventricular surface and weakens towards the deeper layers of parenchyma. Based on the transgenic misexpression<br />
of the dominant negative and the full-length variants of cxcr5 gene in the ventricular zone cells, we propose that cxcr5 is a permissive cue for<br />
proliferative competency of the RGCs. cxcr5 is sufficient to enhance proliferation of RGCs not before but after injury, possibly due to additional<br />
injury-induced factors required in combination with cxcr5 to enhance cell proliferation. Based on the expression of cxcr5 in differentiating neurons<br />
and long-term neurogenesis experiments upon transgenic misexpression, we suggest that cxcr5 is also required for differentiation of RGCs to<br />
neurons. Since cxcr5 is also expressed in neurons, it might have other roles in neurons, such as migration, which are yet to be identified.<br />
to produce clinical ramifications in efforts for regenerative<br />
therapeutic applications for human neurological disorders<br />
or acute injuries.<br />
Methods<br />
Ethics statement<br />
All animal experiments were carried out in accordance<br />
with the recommendations and permits of the Landesdirektion<br />
Dresden (Permit numbers: AZ 24D-9168.11-1/<br />
2008-2, 4 and 14). All surgery was performed under<br />
anesthesia, and all efforts were made to minimize suffering.<br />
Fish were raised and kept at 28°C under a 14-hour<br />
light, 10 hours dark cycle, and fed with brine shrimp<br />
artemia daily as described [51]. Wildtype experimental<br />
animals were adult fish from the gol-b1 line with AB<br />
genetic background [52].<br />
Tissue preparation and cryosectioning<br />
Fish were sacrificed with 1% 3-amino-benzoic acid ethyl<br />
ester (MESAB, Sigma, Schnelldorf, Germany) and the<br />
skull was opened at the hindbrain area with sharp forceps<br />
to enable a better fixation of the tissue. The skull<br />
was partly removed without damaging or relocating the<br />
telencephalon. The head was cut off behind the pectoral<br />
fins with a scalpel and the brains were fixed in 2% paraformaldehyde<br />
(PFA, Sigma, Schnelldorf, Germany) at 4°C<br />
overnight. Heads were washed twice with 0.1 M phosphate<br />
buffer, transferred in 20% sucrose-ethylenediamine-tetra-acetic<br />
acid (EDTA, Sigma, Schnelldorf,<br />
Germany) solution (in 0.1 M phosphate buffer, pH: 7.5)<br />
and incubated overnight at 4°C. Brains were then frozen<br />
in 7.5% gelatin/20% sucrose in 0.1 M phosphate buffer<br />
and cryosectioned at 14-μm thickness using a cryostat.