Falsification Of The Atmospheric CO2 Greenhouse Effects Within ...
Falsification Of The Atmospheric CO2 Greenhouse Effects Within ...
Falsification Of The Atmospheric CO2 Greenhouse Effects Within ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Falsification</strong> <strong>Of</strong> <strong>The</strong> <strong>Atmospheric</strong> CO 2 <strong>Greenhouse</strong> <strong>Effects</strong> . . . 9<br />
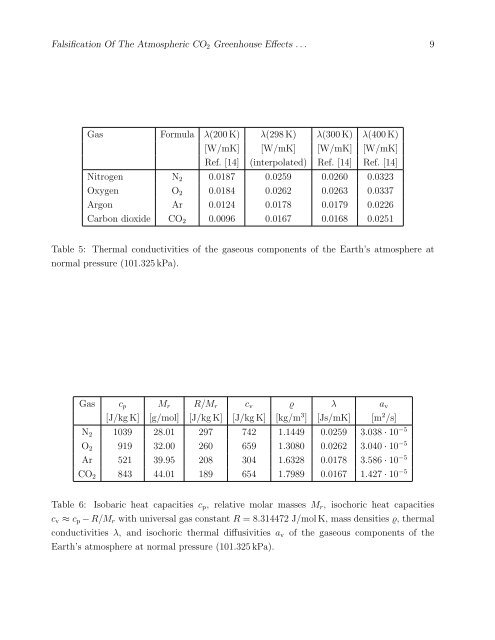
Gas Formula λ(200 K) λ(298 K) λ(300 K) λ(400 K)<br />
[W/mK] [W/mK] [W/mK] [W/mK]<br />
Ref. [14] (interpolated) Ref. [14] Ref. [14]<br />
Nitrogen N 2 0.0187 0.0259 0.0260 0.0323<br />
Oxygen O 2 0.0184 0.0262 0.0263 0.0337<br />
Argon Ar 0.0124 0.0178 0.0179 0.0226<br />
Carbon dioxide CO 2 0.0096 0.0167 0.0168 0.0251<br />
Table 5: <strong>The</strong>rmal conductivities of the gaseous components of the Earth’s atmosphere at<br />
normal pressure (101.325 kPa).<br />
Gas c p M r R/M r c v ϱ λ a v<br />
[J/kg K] [g/mol] [J/kg K] [J/kg K] [kg/m 3 ] [Js/mK] [m 2 /s]<br />
N 2 1039 28.01 297 742 1.1449 0.0259 3.038 · 10 −5<br />
O 2 919 32.00 260 659 1.3080 0.0262 3.040 · 10 −5<br />
Ar 521 39.95 208 304 1.6328 0.0178 3.586 · 10 −5<br />
CO 2 843 44.01 189 654 1.7989 0.0167 1.427 · 10 −5<br />
Table 6: Isobaric heat capacities c p , relative molar masses M r , isochoric heat capacities<br />
c v ≈ c p − R/M r with universal gas constant R = 8.314472 J/mol K, mass densities ϱ, thermal<br />
conductivities λ, and isochoric thermal diffusivities a v of the gaseous components of the<br />
Earth’s atmosphere at normal pressure (101.325 kPa).