Alameda Alliance for Health
Alameda Alliance for Health
Alameda Alliance for Health
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Alameda</strong> <strong>Alliance</strong> <strong>for</strong> <strong>Health</strong><br />
FORMULARY UPDATE<br />
Effective April 15, 2013<br />
<strong>Alameda</strong> <strong>Alliance</strong> <strong>for</strong> <strong>Health</strong> Pharmacy & Therapeutics (P & T) Committee Decisions<br />
The P &T Committee reviewed the efficacy, safety, cost, and utilization profiles of the<br />
following therapeutic categories at the March 7, 2013 meeting:<br />
• Hypnotics /insomnia<br />
• Statins<br />
• Psoriasis topical agents<br />
• Oral agents <strong>for</strong> the treatment of Diabetes<br />
• Atypical Antipsychotics<br />
*The P &T Committee approved the following modifications to the <strong>for</strong>mulary <strong>for</strong> the <strong>Alliance</strong>’s<br />
Medi-Cal, and <strong>Alliance</strong> Group Care programs:<br />
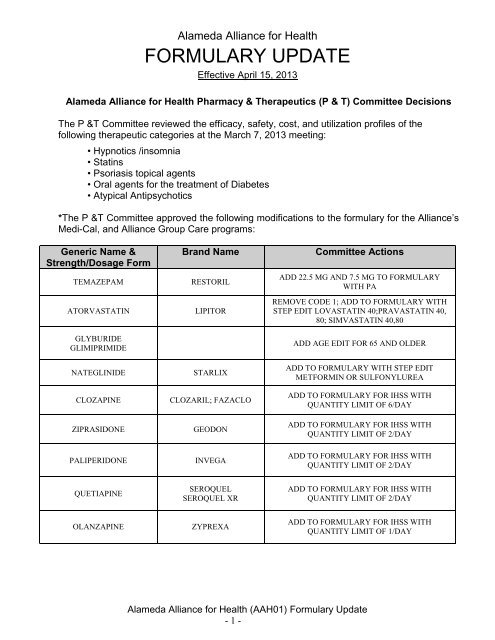
Generic Name &<br />
Strength/Dosage Form<br />
TEMAZEPAM<br />
ATORVASTATIN<br />
GLYBURIDE<br />
GLIMIPRIMIDE<br />
NATEGLINIDE<br />
CLOZAPINE<br />
ZIPRASIDONE<br />
PALIPERIDONE<br />
Brand Name<br />
RESTORIL<br />
LIPITOR<br />
STARLIX<br />
CLOZARIL; FAZACLO<br />
GEODON<br />
INVEGA<br />
Committee Actions<br />
ADD 22.5 MG AND 7.5 MG TO FORMULARY<br />
WITH PA<br />
REMOVE CODE 1; ADD TO FORMULARY WITH<br />
STEP EDIT LOVASTATIN 40;PRAVASTATIN 40,<br />
80; SIMVASTATIN 40,80<br />
ADD AGE EDIT FOR 65 AND OLDER<br />
ADD TO FORMULARY WITH STEP EDIT<br />
METFORMIN OR SULFONYLUREA<br />
ADD TO FORMULARY FOR IHSS WITH<br />
QUANTITY LIMIT OF 6/DAY<br />
ADD TO FORMULARY FOR IHSS WITH<br />
QUANTITY LIMIT OF 2/DAY<br />
ADD TO FORMULARY FOR IHSS WITH<br />
QUANTITY LIMIT OF 2/DAY<br />
QUETIAPINE<br />
SEROQUEL<br />
SEROQUEL XR<br />
ADD TO FORMULARY FOR IHSS WITH<br />
QUANTITY LIMIT OF 2/DAY<br />
OLANZAPINE<br />
ZYPREXA<br />
ADD TO FORMULARY FOR IHSS WITH<br />
QUANTITY LIMIT OF 1/DAY<br />
<strong>Alameda</strong> <strong>Alliance</strong> <strong>for</strong> <strong>Health</strong> (AAH01) Formulary Update<br />
- 1 -
Generic Name &<br />
Strength/Dosage Form<br />
FLUTICASONE<br />
Brand Name<br />
FLOVENT DISKUS<br />
Committee Actions<br />
FORMULARY WITH STEP EDIT QVAR AND<br />
ADVAIR DISKUS<br />
PRIOR AUTHORIZATION GUIDELINES UPDATES<br />
TOPICAL NSAIDS<br />
DICLOFENAC (SOLARAZE)<br />
STEP THERAPY (VARIOUS)<br />
ARIPIPRAZOLE (ABILIFY)<br />
INCRETIN MIMETICS (BYETTA; VICTOZA)<br />
INSULIN DELIVERY SYSTEMS (APIDRA SOLOSTAR; LANTUS SOLOSTAR; HUMALOG<br />
KWIKPEN; NOVOLOG; NOVOLOG RAPID<br />
TOPICAL TACROLIMUSM (PROTOPIC)<br />
TOPICAL PIMECROLIMUS (ELIDEL)<br />
TESTOSTERONE TOPICAL TESTOSTERONE; TESTOSTERONE CYPIONATE;<br />
TESTOSTERONE ENANTHATE<br />
ESTROGEN PATCHES ALORA; CLIMARA, VIVELLE/VIVELLE DOT<br />
EPOETIN ALFA (EPOGEN;PROCRIT)<br />
DARBOEPOETIN ALFA (ARANESP)<br />
EZETIMIBE (ZETIA)<br />
ATORVATATIN LIPITOR<br />
ATOMOXETINE (STRATTERA)<br />
*Note: Drugs removed from the <strong>for</strong>mulary will NOT be grandfathered <strong>for</strong> utilizing<br />
members unless noted otherwise under “Committee Actions.”<br />
<strong>Alameda</strong> <strong>Alliance</strong> <strong>for</strong> <strong>Health</strong> (AAH01) Formulary Update<br />
- 2 -