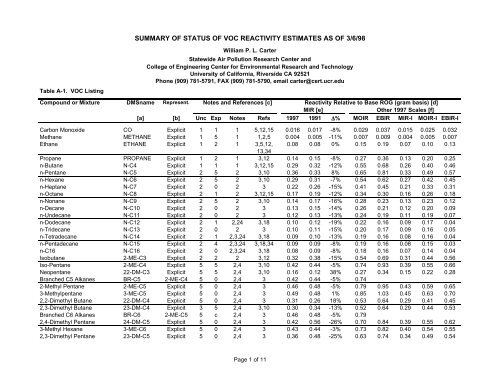
SUMMARY OF STATUS OF VOC REACTIVITY ... - CE-CERT
SUMMARY OF STATUS OF VOC REACTIVITY ... - CE-CERT
SUMMARY OF STATUS OF VOC REACTIVITY ... - CE-CERT
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-1. <strong>VOC</strong> Listing<br />
William P. L. Carter<br />
Statewide Air Pollution Research Center and<br />
College of Engineering Center for Environmental Research and Technology<br />
University of California, Riverside CA 92521<br />
Phone (909) 781-5791, FAX (909) 781-5790, email carter@cert.ucr.edu<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
Carbon Monoxide CO Explicit 1 1 1 5,12,15 0.016 0.017 -8% 0.029 0.037 0.015 0.025 0.032<br />
Methane METHANE Explicit 1 5 1 1,2,5 0.004 0.005 -11% 0.007 0.009 0.004 0.005 0.007<br />
Ethane ETHANE Explicit 1 2 1 3,5,12, 0.08 0.08 0% 0.15 0.19 0.07 0.10 0.13<br />
13,34<br />
Propane PROPANE Explicit 1 2 1 3,12 0.14 0.15 -8% 0.27 0.36 0.13 0.20 0.25<br />
n-Butane N-C4 Explicit 1 1 1 3,12,15 0.29 0.32 -12% 0.55 0.68 0.26 0.40 0.46<br />
n-Pentane N-C5 Explicit 2 5 2 3,10 0.36 0.33 8% 0.65 0.81 0.33 0.49 0.57<br />
n-Hexane N-C6 Explicit 2 5 2 3,10 0.29 0.31 -7% 0.54 0.62 0.27 0.42 0.45<br />
n-Heptane N-C7 Explicit 2 0 2 3 0.22 0.26 -15% 0.41 0.45 0.21 0.33 0.31<br />
n-Octane N-C8 Explicit 2 1 2 3,12,15 0.17 0.19 -12% 0.34 0.30 0.16 0.26 0.18<br />
n-Nonane N-C9 Explicit 2 5 2 3,10 0.14 0.17 -16% 0.28 0.23 0.13 0.23 0.12<br />
n-Decane N-C10 Explicit 2 0 2 3 0.13 0.15 -14% 0.26 0.21 0.12 0.20 0.09<br />
n-Undecane N-C11 Explicit 2 0 2 3 0.12 0.13 -13% 0.24 0.19 0.11 0.19 0.07<br />
n-Dodecane N-C12 Explicit 2 1 2,24 3,18 0.10 0.12 -19% 0.22 0.16 0.09 0.17 0.04<br />
n-Tridecane N-C13 Explicit 2 0 2 3 0.10 0.11 -15% 0.20 0.17 0.09 0.16 0.05<br />
n-Tetradecane N-C14 Explicit 2 1 2,3,24 3,18 0.09 0.10 -13% 0.19 0.16 0.08 0.16 0.04<br />
n-Pentadecane N-C15 Explicit 2 4 2,3,24 3,18,34 0.09 0.09 -8% 0.19 0.16 0.08 0.15 0.03<br />
n-C16 N-C16 Explicit 2 0 2,3,24 3,18 0.08 0.09 -8% 0.18 0.16 0.07 0.14 0.04<br />
Isobutane 2-ME-C3 Explicit 2 2 2 3,12 0.32 0.38 -15% 0.54 0.69 0.31 0.44 0.56<br />
Iso-Pentane 2-ME-C4 Explicit 5 5 2,4 3,10 0.42 0.44 -5% 0.74 0.93 0.39 0.55 0.66<br />
Neopentane 22-DM-C3 Explicit 5 5 2,4 3,10 0.16 0.12 38% 0.27 0.34 0.15 0.22 0.28<br />
Branched C5 Alkanes BR-C5 2-ME-C4 5 0 2,4 3 0.42 0.44 -5% 0.74<br />
2-Methyl Pentane 2-ME-C5 Explicit 5 0 2,4 3 0.46 0.48 -5% 0.79 0.95 0.43 0.59 0.65<br />
3-Methylpentane 3-ME-C5 Explicit 5 0 2,4 3 0.49 0.48 1% 0.85 1.03 0.45 0.63 0.70<br />
2,2-Dimethyl Butane 22-DM-C4 Explicit 5 0 2,4 3 0.31 0.26 18% 0.53 0.64 0.29 0.41 0.45<br />
2,3-Dimethyl Butane 23-DM-C4 Explicit 3 5 2,4 3,10 0.30 0.34 -13% 0.52 0.64 0.29 0.44 0.53<br />
Branched C6 Alkanes BR-C6 2-ME-C5 5 c 2,4 3 0.46 0.48 -5% 0.79<br />
2,4-Dimethyl Pentane 24-DM-C5 Explicit 5 0 2,4 3 0.42 0.56 -26% 0.70 0.84 0.39 0.55 0.62<br />
3-Methyl Hexane 3-ME-C6 Explicit 5 0 2,4 3 0.43 0.44 -3% 0.73 0.82 0.40 0.54 0.55<br />
2,3-Dimethyl Pentane 23-DM-C5 Explicit 5 0 2,4 3 0.36 0.48 -25% 0.63 0.74 0.34 0.49 0.54<br />
Page 1 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
2,2,3-Trimethyl Butane 223TM-C4 Explicit 5 0 2,4 3 0.34 0.42 -18% 0.55 0.68 0.33 0.47 0.57<br />
Branched C7 Alkanes BR-C7 3-ME-C6 5 0 2,4 3 0.43 0.44 -3% 0.73<br />
2-Methyl Heptane 2-ME-C7 Explicit 5 0 2,4 3 0.30 0.30 0% 0.53 0.57 0.29 0.41 0.39<br />
3-Methyl Heptane 3-ME-C7 Explicit 5 0 2,4 3 0.33 0.31 4% 0.56 0.63 0.31 0.44 0.43<br />
4-Methyl Heptane 4-ME-C7 Explicit 5 0 2,4 3 0.35 0.38 -8% 0.59 0.64 0.33 0.45 0.42<br />
2,3-Dimethyl Hexane 23-DM-C6 Explicit 5 0 2,4 3 0.35 0.42 -16% 0.60 0.68 0.33 0.47 0.49<br />
2,4-Dimethyl Hexane 24-DM-C6 Explicit 5 0 2,4 3 0.51 0.48 7% 0.82 0.97 0.48 0.65 0.71<br />
2,5-Dimethyl Hexane 25-DM-C6 Explicit 5 0 2,4 3 0.50 0.52 -3% 0.82 0.99 0.48 0.65 0.74<br />
2,2,4-Trimethyl Pentane 224TM-C5 Explicit 3 2 2,4 3,12 0.33 0.29 12% 0.53 0.61 0.31 0.43 0.48<br />
2,3,4-Trimethyl Pentane 234TM-C5 Explicit 5 0 2,4 3 0.35 0.51 -31% 0.60 0.69 0.33 0.48 0.51<br />
2,2,3,3-Tetramethyl Butane 2233M-C4 Explicit 5 0 2,4 3 0.11 0.11 -1% 0.20 0.23 0.11 0.16 0.18<br />
Branched C8 Alkanes BR-C8 4-ME-C7 5 0 2,4 3 0.35 0.38 -8% 0.59<br />
2,4-Dimethyl Heptane 24-DM-C7 Explicit 8 0 2,4 3 0.44 0.42 5% 0.72 0.81 0.42 0.57 0.58<br />
4-Ethyl Heptane 4-ET-C7 Explicit 8 0 2,4 3 0.34 0.36 -6% 0.57 0.61 0.32 0.43 0.38<br />
2,2,5-Trimethyl Hexane 225TM-C6 Explicit 8 0 2,4 3 0.33 0.31 8% 0.56 0.61 0.31 0.43 0.42<br />
Branched C9 Alkanes BR-C9 4-ET-C7 8 0 2,4,7 3 0.34 0.36 -6% 0.57<br />
3,4-Propyl Heptane 4-PR-C7 Explicit 8 0a 2,4 3,27 0.30 0.32 -7% 0.49 0.53 0.28 0.38 0.32<br />
Branched C10 Alkanes BR-C10 4-PR-C7 8 a 2,4,7 27 0.30 0.32 -7% 0.49<br />
3,5-Diethyl Heptane 35-DE-C7 Explicit 8 0a 2,4 3,27 0.45 0.37 21% 0.70 0.81 0.43 0.56 0.57<br />
Branched C11 alkanes BR-C11 35-DE-C7 8 a 2,4,7 27 0.45 0.37 21% 0.70<br />
2,6-Diethyl Octane 36-DE-C8 Explicit 8 0a 2,4 3,27 0.34 0.39 -13% 0.55 0.63 0.32 0.44 0.42<br />
Branched C12 Alkanes BR-C12 36-DE-C8 8 a 2,4,7 27 0.34 0.39 -13% 0.55<br />
3,7-Diethyl Nonane 37-DE-C9 Explicit 8 0a 2,4 3,27 0.34 0.30 11% 0.53 0.58 0.32 0.42 0.37<br />
Branched C13 Alkanes BR-C13 37-DE-C9 8 a 2,4,7 27 0.34 0.30 11% 0.53<br />
3,8-Diethyl Decane 38DE-C10 Explicit 8 0 2,4 3 0.20 0.24 -16% 0.34 0.37 0.19 0.29 0.24<br />
Branched C14 Alkanes BR-C14 38DE-C10 8 0 2,4,7 27 0.20 0.24 -16% 0.34<br />
3,9-Diethyl Undecane 39DE-C11 Explicit 8 0 2,4 3 0.17 0.22 -22% 0.30 0.32 0.16 0.25 0.19<br />
Branched C15 Alkanes BR-C15 39DE-C11 8 0 2,4,7 0.17 0.22 -22% 0.30<br />
Branched C16 Alkanes BR-C16 39DE-C11 8 0 2,4,7 0.16 0.21 -22% 0.28<br />
Branched C17 Alkanes BR-C17 39DE-C11 8 0 2,4,7 0.15 0.19 -22% 0.26<br />
Branched C18 Alkanes BR-C18 39DE-C11 8 0 2,4,7 0.14 0.18 -22% 0.25<br />
Cyclopropane CYCC3 Explicit 5 0 2,5 3 0.02 0.05 0.06 0.02 0.03 0.04<br />
Cyclobutane CYCC4 Explicit 5 0 2,5 3 0.28 0.55 0.70 0.26 0.37 0.43<br />
Cyclopentane CYCC5 Explicit 3 0 2,6 3 0.65 0.75 -14% 1.14 1.39 0.61 0.84 0.99<br />
Methylcyclopentane ME-CYCC5 Explicit 3 0 2,6 3 0.80 0.89 -11% 1.26 1.48 0.76 0.96 1.04<br />
Cyclohexane CYCC6 Explicit 3 1b 2,6 3,31 0.41 0.41 2% 0.71 0.79 0.39 0.54 0.56<br />
C6 Cycloalkanes CYC-C6 CYCC6 3 c 2,6 0.41 0.41 2% 0.71<br />
Page 2 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
Methylcyclohexane ME-CYCC6 Explicit 3 5 2,6 3,9 0.48 0.58 -18% 0.78 0.85 0.45 0.59 0.56<br />
1,3-Dimeth. Cyclopentane 13DMCYC5 Explicit 5 0 2,6 3 0.73 0.81 -9% 1.12 1.28 0.70 0.86 0.87<br />
Ethyl Cyclopentane ET-CYCC5 Explicit 5 0 2,6 3 0.73 0.73 -1% 1.14 1.32 0.69 0.85 0.86<br />
C7 Cycloalkanes CYC-C7 ME-CYCC6 5 0 2,6 0.48 0.58 -18% 0.78<br />
Propyl Cyclopentane PR-CYCC5 Explicit 6 0 2,6 3 0.60 0.65 -8% 0.95 1.06 0.57 0.71 0.66<br />
Ethylcyclohexane ET-CYCC6 Explicit 6 0 2,6 3 0.42 0.61 -32% 0.69 0.72 0.40 0.52 0.44<br />
1,3-Dimethyl Cyclohexane 13DMCYC6 Explicit 6 0 2,6 3 0.47 0.66 -29% 0.75 0.82 0.45 0.58 0.53<br />
C8 Cycloalkanes CYC-C8 ET-CYCC6 6 0 2,6 0.42 0.61 -32% 0.69<br />
1-Eth.-4-Meth. Cyclohex. 1E4MCYC6 Explicit 6 0 2,6 3 0.46 0.73 -36% 0.74 0.79 0.44 0.56 0.48<br />
C9 Cycloalkanes CYC-C9 1E4MCYC6 6 0 2,6,7 0.46 0.73 -36% 0.74<br />
1,3-Diethyl-Cyclohexane 13DECYC6 Explicit 8 0a 2,6 3,27 0.38 0.56 -34% 0.61 0.63 0.36 0.46 0.37<br />
C10 Cycloalkanes CYC-C10 13DECYC6 8 a 2,6,7 27 0.38 0.56 -34% 0.61<br />
13-Dieth-5-Me. Cyclohex. 13E5MCC6 Explicit 8 0a 2,6 3,27 0.43 0.60 -29% 0.65 0.73 0.41 0.51 0.46<br />
C11 Cycloalkanes CYC-C11 13E5MCC6 8 a 2,6,7 27 0.43 0.60 -29% 0.65<br />
1,3,5-Triethyl Cyclohex. 135ECYC6 Explicit 8 0a 2,6 3,27 0.40 0.53 -24% 0.62 0.70 0.39 0.49 0.43<br />
Hexyl Cyclohexane C6-CYCC6 Explicit 8c 1b 2,6 27,31 0.20 0.36 0.36 0.19 0.28 0.18<br />
C12 Cycloalkanes CYC-C12 135ECYC6 8 a 2,6,7 27 0.40 0.53 -24% 0.62<br />
13-Dieth-5-Pent Cyclohx. 13E5PCC6 Explicit 8 0a 2,6 3,27 0.37 0.45 -18% 0.57 0.64 0.35 0.45 0.39<br />
C13 Cycloalkanes CYC-C13 13E5PCC6 8 a 2,6,7 27 0.37 0.45 -18% 0.57<br />
13-Diprop-5-Eth Cyclohx. 13P5ECC6 Explicit 8 0a 2,6 3,27 0.34 0.39 -12% 0.53 0.58 0.33 0.42 0.35<br />
Octyl Cyclohexane C8-CYCC6 Explicit 8c 2b 2,6 3,27,31 0.15 0.28 0.28 0.15 0.23 0.13<br />
C14 Cycloalkanes CYC-C14 13P5ECC6 8 a 2,6,7 27 0.34 0.39 -12% 0.53<br />
135-Tripropyl Cyclohex. 135PCYC6 Explicit 8 0 2,6 3 0.32 0.34 -7% 0.49 0.53 0.30 0.40 0.32<br />
C15 Cycloalkanes CYC-C15 135PCYC6 8 0 2,6,7 0.32 0.34 -7% 0.49<br />
C9 Bicycloalkanes BCYC-C9 1E4MCYC6 10 0 2,6,7 0.47 0.74 -36% 0.75<br />
C10 Bicycloalkanes BCYC-C10 13DECYC6 10 0 2,6,7 0.38 0.57 -34% 0.61<br />
C11 Bicycloalkanes BCYC-C11 13E5MCC6 10 0 2,6,7 0.43 0.61 -29% 0.66<br />
C12 Bicycloalkanes BCYC-C12 135ECYC6 10 0 2,6,7 0.41 0.54 -24% 0.63<br />
C13 Bicycloalkanes BCYC-C13 13E5PCC6 10 0 2,6,7 0.37 0.46 -18% 0.57<br />
C14 Bicycloalkanes BCYC-C14 13P5ECC6 10 0 2,6,7 0.35 0.39 -12% 0.53<br />
C15 Bicycloalkanes BCYC-C15 135PCYC6 10 0 2,6,7 0.32 0.34 -7% 0.50<br />
Ethene ETHENE Explicit 2b 1 1 10,12,15 2.05 2.31 -11% 2.31 2.62 2.01 2.23 2.59<br />
Propene PROPENE Explicit 2 1 1 10,12,15 2.72 2.98 -9% 2.90 3.40 2.74 2.86 3.23<br />
1-Butene 1-BUTENE Explicit 2 3 8 9b,10,12 2.61 2.82 -8% 2.88 3.43 2.63 2.72 3.06<br />
3-Methyl-1-Butene 3M-1-BUT Explicit 3 0 8 1.78 1.97 -10% 2.01 2.29 1.78 1.83 1.88<br />
1-Pentene 1-PENTEN Explicit 3 0 8,9 1.78 1.97 -10% 2.01 2.29 1.78 1.83 1.88<br />
C5 Terminal Alkanes C5-OLE1 1-PENTEN 3 0 8,9 1.78 1.97 -10% 2.01<br />
Page 3 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
1-Hexene 1-HEXENE Explicit 3 3 8,9 9b 1.40 1.40 0% 1.59 1.79 1.40 1.44 1.41<br />
C6 Terminal Alkanes C6-OLE1 1-HEXENE 3 c 8,9 1.40 1.40 0% 1.59<br />
1-Heptene 1-C7-OLE Explicit 5 0 8,9 1.12 1.10 2% 1.28 1.43 1.12 1.15 1.08<br />
C7 Terminal Alkanes C7-OLE1 1-C7-OLE 5 0 8,9 1.12 1.10 2% 1.28<br />
1-Octene 1-C8-OLE Explicit 5 0 8,9 0.89 0.85 5% 1.04 1.14 0.88 0.92 0.80<br />
C8 Terminal Alkanes C8-OLE1 1-C8-OLE 5 0 8,9 0.89 0.85 5% 1.04<br />
1-Nonene 1-C9-OLE Explicit 5 0 8,9 0.74 0.71 5% 0.87 0.94 0.73 0.76 0.63<br />
C9 Terminal Alkanes C9-OLE1 1-C9-OLE 5 0 8,9 0.74 0.71 5% 0.87<br />
Isobutene ISOBUTEN Explicit 3 2 8,10 10,12 1.42 1.68 -16% 1.32 1.42 1.47 1.52 1.64<br />
2-Methyl-1-Butene 2M-1-BUT Explicit 5 0 8,11 1.32 1.55 -15% 1.38 1.53 1.34 1.44 1.54<br />
2-Methyl-1-Pentene 2M-1-PEN 2M-1-BUT 5 0 8,11 1.10 1.15<br />
233-Trimethyl-1-butene 233TM-1B 2M-1-BUT 5 0 8,11 0.95 0.99<br />
trans-2-Butene T-2-BUTE Explicit 2 1 1 10,12,15 3.24 3.15 3% 3.14 3.63 3.33 3.46 3.88<br />
cis-2-Butene C-2-BUTE Explicit 2 5 1 10 3.11 3.15 -1% 3.11 3.66 3.20 3.31 3.71<br />
C4 Internal Alkenes C4-OLE2 T-2-BUTE 2 a 1 3.24 3.15 3% 3.14<br />
trans-2-Pentene T-2-PENT 2-C5-OLE 3 0 8,9,11 2.84 2.81<br />
cis-2-Pentene C-2-PENT 2-C5-OLE 3 0 8,9,11 2.84 2.81<br />
2-Methyl-2-Butene 2M-2-BUT Explicit 3 0 8,9,11 2.74 2.03 35% 2.39 2.50 2.87 3.01 3.30<br />
2-Pentenes 2-C5-OLE Explicit 3 0 8,9,11 2.84 2.79 2% 2.81 3.21 2.90 2.99 3.25<br />
C5 Internal Alkenes C5-OLE2 2-C5-OLE 5 0 7,8,9,11 2.84 2.79 2% 2.81<br />
2-Methyl-2-Pentene 2M-2-PEN 2M-2-BUT 5 0 8,9,11 2.29 1.98<br />
2,3-Dimethyl-2-Butene 23M2-BUT Explicit 5 0 8,9,11 2.66 2.09 1.96 2.81 3.06 3.42<br />
2-Hexenes 2-C6-OLE Explicit 5 0 8,9,11 2.27 2.12 7% 2.27 2.57 2.33 2.41 2.57<br />
C6 Internal Alkenes C6-OLE2 2-C6-OLE 5 0 7,8,9,11 2.27 2.12 7% 2.27<br />
2-Heptenes 2-C7-OLE Explicit 5 0 8,9,11 1.89 1.75 8% 1.89 2.13 1.94 2.01 2.11<br />
C7 Internal Alkenes C7-OLE2 2-C7-OLE 5 0 7,8,9,11 1.89 1.75 8% 1.89<br />
3-Octenes 3-C8-OLE Explicit 5 0 8,9,11 1.81 1.67 8% 1.86 2.13 1.83 1.90 1.98<br />
C8 Internal Alkenes C8-OLE2 3-C8-OLE 5 0 7,8,9,11 1.81 1.67 8% 1.86<br />
3-Nonenes 3-C9-OLE Explicit 5 0 8,9,11 1.56 1.45 8% 1.62 1.83 1.59 1.66 1.70<br />
C9 Internal Alkenes C9-OLE2 3-C9-OLE 5 0 7,8,9,11 1.56 1.45 8% 1.62<br />
C10 3-Alkenes 3C10-OLE Explicit 5 0 8,9,11 1.38 1.28 7% 1.42 1.60 1.40 1.46 1.48<br />
C10 Internal Alkenes C10-OLE2 3C10-OLE 5 0 7,8,9,11 1.38 1.28 7% 1.42<br />
C11 3-Alkenes 3C11-OLE Explicit 5 0 8,9,11 1.24 1.15 8% 1.28 1.45 1.26 1.32 1.33<br />
C11 Internal Alkenes C11-OLE2 3C11-OLE 5 0 7,8,9,11 1.24 1.15 8% 1.28<br />
C12 2-Alkenes 2C12-OLE 3C12-OLE 5 0 8,9,11 1.13 1.17<br />
C12 3-Alkenes 3C12-OLE Explicit 5 0 8,9,11 1.13 1.05 8% 1.17 1.33 1.15 1.21 1.22<br />
C12 Internal Alkenes C12-OLE2 3C12-OLE 5 0 7,8,9,11 1.13 1.05 8% 1.17<br />
Page 4 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
C13 3-Alkenes 3C13-OLE Explicit 5 0 8,9,11 1.04 0.96 8% 1.07 1.23 1.05 1.12 1.12<br />
C13 Internal Alkenes C13-OLE2 3C13-OLE 5 0 7,8,9,11 1.04 0.96 8% 1.07<br />
1,3-Butadiene 13-BUTDE Explicit 10 0 12 3.08 3.45 -11% 3.28 3.91 3.13 3.20 3.62<br />
Cyclopentadiene CYC-PNDE 2-C5-OLE 10 0 12 3.01 2.96 2% 2.98<br />
Isoprene ISOPRENE Explicit 2 1 13 32,33 2.30 2.87 -20% 2.46 2.91 2.36 2.47 2.80<br />
Cyclopentene CYC-PNTE Explicit 5 0 14 2.21 2.43 -9% 1.94 1.99 2.30 2.49 2.74<br />
Cyclohexene CYC-HEXE Explicit 5 0 14 1.40 1.79 -22% 1.52 1.81 1.42 1.51 1.62<br />
a-Pinene A-PINENE Explicit 3 3 14,15 31 0.96 1.04 -8% 0.95 1.06 0.97 1.05 1.06<br />
b-Pinene B-PINENE Explicit 3 3 14,15 31 0.47 1.40 -66% 0.55 0.60 0.48 0.54 0.47<br />
d-Limonene D-LIMONE Explicit 4 3 14,15 31 0.72 0.74 0.81 0.73 0.80 0.78<br />
3-Carene 3-CARENE Explicit 4 3 14,15 31 0.60 1.04 -42% 0.72 0.82 0.60 0.68 0.64<br />
Sabinene SABINENE Explicit 5 0 14,15 0.53 0.57 0.61 0.53 0.59 0.53<br />
C6 Cyclic or di-olefins C6-OL2D 2-C6-OLE 10 0 7,12 2.33 2.17 7% 2.32<br />
C7 Cyclic or di-olefins C7-OL2D 2-C7-OLE 10 0 7,12 1.93 1.79 8% 1.93<br />
C8 Cyclic or di-olefins C8-OL2D 3-C8-OLE 10 0 7,12 1.84 1.70 8% 1.89<br />
C9 Cyclic or di-olefins C9-OL2D 3-C9-OLE 10 0 7,12 1.59 1.47 8% 1.65<br />
C10 Cyclic or di-olefins C10-OL2D 3C10-OLE 10 0 7,12 1.40 1.30 7% 1.44<br />
C11 Cyclic or di-olefins C11-OL2D 3C11-OLE 10 0 7,12 1.26 1.17 8% 1.30<br />
C12 Cyclic or di-olefins C12-OL2D 3C12-OLE 10 0 7,12 1.14 1.06 8% 1.19<br />
C13 Cyclic or di-olefins C13-OL2D 3C13-OLE 10 0 7,12 1.05 0.97 8% 1.09<br />
Benzene BENZENE Explicit 5 1d 16 9a,12,22 0.20 0.13 46% 0.17 0.07 0.18 0.18 0.09<br />
Toluene TOLUENE Explicit 3 1e 16 9a,10, 1.26 0.86 46% 0.87 0.24 1.20 1.06 0.58<br />
12,15,22<br />
Ethyl Benzene C2-BENZ Explicit 3 2e 16 12,22 0.55 0.85 -35% 0.24 -0.35 0.53 0.46 0.06<br />
n-Propyl Benzene N-C3-BEN Explicit 5 0 17 0.44 0.67 -34% 0.18 -0.30 0.42 0.36 0.03<br />
Isopropyl Benzene I-C3-BEN Explicit 5 0 17 0.46 0.71 -34% 0.19 -0.30 0.44 0.39 0.04<br />
C9 Monosub. Benzenes C9-BEN1 TOLUENE 5 0 17 0.49 0.66 -26% 0.21<br />
s-Butyl Benzene S-C4-BEN Explicit 5 0 17 0.39 0.60 -34% 0.16 -0.27 0.38 0.33 0.03<br />
n-Butyl Benzene N-C4-BEN TOLUENE 5 0 17 0.44 0.19<br />
C10 Monosub. Benzenes C10-BEN1 TOLUENE 5 0 17 0.44 0.59 -26% 0.19<br />
C11 Monosub. Benzenes C11-BEN1 TOLUENE 5 0 17 0.40 0.54 -26% 0.17<br />
C12 Monosub. Benzenes C12-BEN1 TOLUENE 5 0 17 0.36 0.49 -26% 0.15<br />
C13 Monosub. Benzenes C13-BEN1 TOLUENE 5 0 17 0.33 0.45 -26% 0.14<br />
o-Xylene O-XYLENE Explicit 3 2e 16 10,12,22 2.08 2.05 2% 1.63 1.23 2.07 1.94 1.58<br />
p-Xylene P-XYLENE Explicit 3 2e 16 12,22 0.71 2.09 -66% 0.46 0.04 0.71 0.68 0.38<br />
m-Xylene M-XYLENE Explicit 3 1e 16 9,10,12,<br />
15,22<br />
3.49 2.58 35% 2.75 2.38 3.56 3.31 2.97<br />
Page 5 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
C8 Disub. Benzenes C8-BEN2 M-XYLENE 3 c 18 3.49 2.58 35% 2.75<br />
C9 Disub. Benzenes C9-BEN2 M-XYLENE 5 0 18 3.08 2.28 35% 2.42<br />
C10 Disub. Benzenes C10-BEN2 M-XYLENE 5 0 18 2.76 2.04 35% 2.17<br />
C11 Disub. Benzenes C11-BEN2 M-XYLENE 5 0 18 2.50 1.85 35% 1.96<br />
C12 Disub. Benzenes C12-BEN2 M-XYLENE 5 0 18 2.28 1.69 35% 1.79<br />
C13 Disub. Benzenes C13-BEN2 M-XYLENE 5 0 18 2.10 1.65<br />
1,3,5-Trimethyl Benzene 135-TMB Explicit 4 2e 16 9a,10, 3.37 3.20 5% 2.58 2.30 3.54 3.42 3.27<br />
12,22<br />
1,2,3-Trimethyl Benzene 123-TMB Explicit 4 2e 16 12,22 3.03 2.80 8% 2.36 2.08 3.13 2.98 2.77<br />
1,2,4-Trimethyl Benzene 124-TMB Explicit 4 2e 16 12,22 1.31 2.80 -53% 0.96 0.61 1.36 1.31 1.06<br />
C9 Trisub. Benzenes C9-BEN3 135-TMB 4 c 18 3.37 3.20 5% 2.58<br />
C10 Trisub. Benzenes C10-BEN3 135-TMB 5a 0 18 3.02 2.87 5% 2.31<br />
C11 Trisub. Benzenes C11-BEN3 135-TMB 5a 0 18 2.73 2.60 5% 2.09<br />
C12 Trisub. Benzenes C12-BEN3 135-TMB 5a 0 18 2.49 2.37 5% 1.91<br />
C13 Trisub. Benzenes C13-BEN3 135-TMB 5a 0 18 2.30 1.76<br />
C10 Tetrasub. Benzenes C10-BEN4 135-TMB 5a 0 18 3.02 2.87 5% 2.31<br />
C11 Tetrasub. Benzenes C11-BEN4 135-TMB 5a 0 18 2.73 2.60 5% 2.09<br />
C12 Tetrasub. Benzenes C12-BEN4 135-TMB 5a 0 18 2.49 2.37 5% 1.91<br />
C11 Pentasub. Benzenes C11-BEN5 135-TMB 5a 0 18 2.73 2.60 5% 2.09<br />
C11 Pentasub. Benzenes C12-BEN5 135-TMB 5a 0 18 2.49 2.37 5% 1.91<br />
C12 Hexaasub. Benzenes C12-BEN6 135-TMB 5a 0 18 2.49 2.37 5% 1.91<br />
Tetralin TETRALIN Explicit 5 3f 19 9a,10 0.26 0.30 -14% 0.08 -0.27 0.26 0.24 -0.05<br />
Indan INDAN TETRALIN 7 0 20 0.29 0.34 -14% 0.09<br />
C11 Tetralin or Indane C11-TET TETRALIN 7 0 20 0.23 0.27 -14% 0.08<br />
Naphthalene NAPHTHAL Explicit 5 3f 19 9a,10 0.31 0.37 -17% 0.04 -0.46 0.30 0.24 -0.17<br />
Methyl Naphthalenes ME-NAPH Explicit 7 0 21 0.78 1.04 -24% 0.46 0.04 0.80 0.70 0.31<br />
C12 Monosub. Naphth. C12-NAP1 ME-NAPH 7 0 21 0.71 0.94 -24% 0.42<br />
C13 Monosub. Naphth. C13-NAP1 ME-NAPH 7 0 21 0.66 0.87 -24% 0.38<br />
2,3-Dimethyl Naphth. 23-DMN Explicit 5 3f 19 9a,10 1.19 1.62 -27% 0.81 0.46 1.24 1.13 0.77<br />
Dimethyl Naphthalenes DM-NAPH 23-DMN 6 0 22 1.19 1.62 -27% 0.81<br />
C12 Disub. Naphthalenes C12-NAP2 23-DMN 6 0 22 1.19 1.62 -27% 0.81<br />
C13 Disub. Naphthalenes C13-NAP2 23-DMN 6 0 8 1.09 1.49 -27% 0.74<br />
C13 Trisub. Naphthalenes C13-NAP3 23-DMN 6 0 8 1.09 1.49 -27% 0.74<br />
Styrene STYRENE Explicit 5 7 23 0.56 0.70 -20% -0.38 -2.28 0.56 0.16 -1.14<br />
a-Methyl Styrene AME-STYR STYRENE 6 0 8 0.49 0.62 -20% -0.33<br />
C9 Styrenes C9-STYR STYRENE 6 0 8 0.49 0.62 -20% -0.33<br />
C10 Styrenes C10-STYR STYRENE 6 0 8 0.44 0.55 -20% -0.30<br />
Page 6 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
Acetylene A<strong>CE</strong>TYLEN Explicit 9 1 24-26 24 0.09 0.16 -45% 0.13 0.15 0.08 0.11 0.12<br />
Methyl Acetylene ME-ACTYL Explicit 10 0 27 1.23 1.30 -5% 1.97 2.48 1.15 1.44 1.71<br />
Ethyl Acetylene ET-ACTYL 1-BUTENE 10 0 27 2.71 2.93 -8% 2.99<br />
Methanol MEOH Explicit 2b 2 1 10,12 0.16 0.18 -12% 0.20 0.22 0.15 0.18 0.20<br />
Ethanol ETOH Explicit 1 2 1 12 0.42 0.42 -1% 0.66 0.79 0.39 0.48 0.51<br />
n-Propyl Alcohol N-C3-OH Explicit 2 0 1 0.67 0.71 -6% 1.02 1.24 0.62 0.74 0.81<br />
Isopropyl Alcohol I-C3-OH Explicit 2 1 1 12,31 0.18 0.17 5% 0.27 0.35 0.17 0.25 0.30<br />
Isobutyl Alcohol I-C4-OH Explicit 3 0 28 0.55 0.61 -9% 0.80 0.99 0.52 0.64 0.71<br />
n-Butyl Alcohol N-C4-OH Explicit 5 0 28 0.79 0.85 -7% 1.15 1.40 0.74 0.88 0.98<br />
t-Butyl Alcohol T-C4-OH Explicit 2 1 28 20 0.10 0.13 -25% 0.15 0.18 0.09 0.13 0.15<br />
s-Butyl Alcohol S-C4-OH Explicit 3 0 28 0.39 0.85 -54% 0.62 0.78 0.37 0.50 0.57<br />
1-Octyl Alcohol 1-C8-OH Explicit 8c 1b 29-31 31 0.42 0.62 0.74 0.41 0.52 0.54<br />
2-Octyl Alcohol 2-C8-OH Explicit 8c 1b 29-31 31 0.22 0.37 0.43 0.21 0.31 0.30<br />
3-Octyl Alcohol 3-C8-OH Explicit 8c 1b 29-31 31 0.22 0.36 0.42 0.21 0.31 0.30<br />
Pentyl Alcohol C5OH Explicit 5 0 28,32 0.60 0.50 19% 0.91 1.13 0.57 0.71 0.82<br />
1-Heptanol 1-C7OH Explicit 6 0 28,32 0.45 0.36 23% 0.69 0.85 0.43 0.56 0.63<br />
2-Ethyl-1-Hexanol 2-ETC6OH Explicit 6 0 28,32 0.42 0.39 7% 0.66 0.81 0.40 0.54 0.60<br />
Ethylene Glycol ET-GLYCL Explicit 3 0 1 0.56 0.61 -8% 0.83 1.00 0.52 0.61 0.66<br />
Propylene Glycol PR-GLYCL Explicit 1 1 24 23 0.61 0.49 24% 0.84 1.05 0.58 0.69 0.79<br />
1,2-Butandiol 12-C4OH2 Explicit 2 0 28 0.41 0.43 -4% 0.60 0.75 0.39 0.49 0.55<br />
1,2-Dihydroxy Hexane C6-GLYCL Explicit 5 0 28,32 0.41 0.43 -4% 0.59 0.73 0.40 0.49 0.55<br />
Dimethyl Ether ME-O-ME Explicit 2 4 33 12 0.22 0.24 -9% 0.43 0.60 0.22 0.35 0.51<br />
Diethyl Ether ET-O-ET Explicit 3 1b 29,30,31 31 0.91 0.84 8% 1.20 1.43 0.90 1.10 1.35<br />
Methyl t-Butyl Ether MTBE Explicit 1 2 33 12 0.18 0.20 -10% 0.31 0.41 0.17 0.27 0.34<br />
Ethyl Isopropyl Ether S-098106 Explicit 4 0 28 0.89 0.85 4% 1.12 1.36 0.89 1.07 1.33<br />
Alpha Methyl Tetrahydrofuran AM-THF Explicit 5 0 29 1.27 1.70 2.11 1.26 1.45 1.74<br />
Ethyl t-Butyl Ether ETBE Explicit 5 7 28 0.53 0.63 -16% 0.74 0.92 0.52 0.68 0.85<br />
2-Butyltetrahydrofuran S-098108 Explicit 6 0 27 0.90 0.77 18% 1.24 1.55 0.88 1.05 1.24<br />
Dibutyl Ether S-098107 Explicit 5 0 28 0.71 0.24 191% 1.03 1.26 0.68 0.82 0.91<br />
2-Methoxy-Ethanol MEO-ETOH Explicit 5 0 28 0.75 1.21 -38% 0.94 1.13 0.74 0.85 0.99<br />
2-Ethoxy-Ethanol ETO-ETOH Explicit 4 2 33 12 0.90 1.05 -14% 1.08 1.29 0.90 1.02 1.21<br />
1-Methoxy-2-Propanol MEOC3OH Explicit 5 1b 29,31 31 0.59 0.81 1.03 0.58 0.70 0.84<br />
1-Ethoxy-2-Propanol ETOC3OH Explicit 5 0 28 0.57 0.64 -11% 0.79 0.96 0.56 0.68 0.80<br />
2-Butoxy-Ethanol BUO-ETOH Explicit 9c 1b 29,31 31 0.57 0.47 22% 0.81 0.96 0.55 0.66 0.72<br />
2-(2-Ethoxyethoxy) Ethanol CARBITOL Explicit 4 2 33 12 0.59 0.41 43% 0.76 0.93 0.59 0.69 0.80<br />
2-(2-Butoxyethoxy)-Ethanol C8-<strong>CE</strong>LSV Explicit 6 0 28 0.46 0.27 72% 0.61 0.72 0.46 0.54 0.58<br />
Methyl Acetate ME-A<strong>CE</strong>T Explicit 1 1 24 19 0.03 0.03 -1% 0.06 0.08 0.02 0.04 0.06<br />
Page 7 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
Methyl Acrylate ME-ACRYL 1-BUTENE 10 0 27 1.70 1.84 -8% 1.88<br />
Vinyl Acetate VIN-A<strong>CE</strong>T 1-BUTENE 10 0 27 1.70 1.84 -8% 1.88<br />
Ethyl Acetate ET-A<strong>CE</strong>T Explicit 1 1 30,34 31 0.17 0.10 74% 0.29 0.38 0.16 0.21 0.25<br />
Ethyl Acrylate ET-ACRYL 1-BUTENE 10 0 27 1.46 1.58 -8% 1.62<br />
Propyl Acetate PR-A<strong>CE</strong>T Explicit 10 0 27,32 0.28 0.29 -5% 0.50 0.62 0.26 0.37 0.43<br />
Methyl Isobutyrate ME-IBUAT Explicit 8c 1b 29-31 31 0.18 0.06 196% 0.31 0.39 0.17 0.24 0.28<br />
Isopropyl Acetate IPR-A<strong>CE</strong>T Explicit 5 0 29 0.29 0.27 5% 0.43 0.53 0.28 0.37 0.45<br />
n-Butyl Acetate BU-A<strong>CE</strong>T Explicit 4 1 30,35 31 0.24 0.21 12% 0.42 0.48 0.22 0.31 0.31<br />
Isobutyl Acetate IBU-A<strong>CE</strong>T Explicit 10 0 27,32 0.33 0.35 -4% 0.59 0.71 0.31 0.44 0.49<br />
t-Butyl Acetate TBU-A<strong>CE</strong>T Explicit 2 1 24 28 0.04 0.06 0.08 0.03 0.05 0.06<br />
2-Ethoxyethyl Acetate CSV-A<strong>CE</strong>T Explicit 10 0 27 0.42 0.43 -2% 0.63 0.77 0.41 0.52 0.61<br />
Isobutyl Isobutyrate IBU-IBTR Explicit 10 0 27,32 0.23 0.23 -3% 0.42 0.48 0.21 0.32 0.34<br />
Isoamyl Isobutyrate IC5IBUAT Explicit 10 0 27,32 0.30 0.33 -9% 0.50 0.61 0.29 0.41 0.48<br />
Dimethyl Succinate DBE-4 Explicit 3 1 24 26 0.05 0.11 0.14 0.05 0.09 0.10<br />
Dimethyl Glutarate DBE-5 Explicit 4 1 24 26 0.10 0.20 0.20 0.09 0.15 0.13<br />
Dimethyl Adipate DBE-6 Explicit 5 0 24 26 0.33 0.50 0.52 0.30 0.37 0.31<br />
Propylene Carbonate PC Explicit 3 1 24 20 0.11 0.14 0.15 0.10 0.12 0.12<br />
Propylene Glycol Methyl Ether PGME-ACT Explicit 8c 1b 29-31 31 0.30 0.43 0.55 0.31 0.43 0.55<br />
Acetate<br />
Subst. C7 Ester (C12) S-098116 Explicit 10 0 7,27 0.22 0.25 -9% 0.38 0.46 0.21 0.32 0.36<br />
Subst. C9 Ester (C12) S-098117 Explicit 10 0 7,27 0.22 0.24 -9% 0.36 0.44 0.21 0.31 0.35<br />
Ethylene Oxide ETOX Explicit 10 0 27 0.01 0.01 -13% 0.02 0.03 0.01 0.02 0.02<br />
Propylene Oxide PROX Explicit 10 0 27 0.10 0.10 2% 0.19 0.24 0.09 0.12 0.15<br />
Furan FURAN M-XYLENE 10 3f 27,36 9a 5.44 4.03 35% 4.28<br />
Formaldehyde FORMALD Explicit 1b 1 1 5,10,12, 1.62 2.26 -28% 1.16 1.01 1.71 1.63 1.60<br />
15,16,22<br />
Acetaldehyde A<strong>CE</strong>TALD Explicit 1 1 1 5,10,12, 1.54 1.75 -12% 1.67 2.10 1.57 1.56 1.71<br />
15,16,22<br />
C3 Aldehydes PROPALD Explicit 3 5 37 5,10 1.85 2.07 -11% 2.05 2.55 1.86 1.86 2.03<br />
C4 Aldehydes C4-RCHO PROPALD 5 0 8 1.48 1.67 -11% 1.65<br />
C5 Aldehydes C5-RCHO PROPALD 5 0 8 1.24 1.40 -11% 1.38<br />
C6 Aldehydes C6-RCHO PROPALD 6 0 8 1.07 1.20 -11% 1.19<br />
C7 Aldehydes C7-RCHO PROPALD 6 0 8 0.94 1.05 -11% 1.04<br />
C8 Aldehydes C8-RCHO PROPALD 6 0 8 0.83 0.94 -11% 0.93<br />
Glyoxal GLYOXAL Explicit 9 0 25,26,37 5 0.51 0.70 -27% 0.43 0.46 0.54 0.56 0.57<br />
Acrolein ACROLEIN Explicit 3 3 40 9a 0.89 2.14 -58% 0.96 1.22 0.91 0.94 0.98<br />
Methacrolein METHACRO Explicit 4 3 13 32 1.31 1.30 1.59 1.36 1.36 1.46<br />
Page 8 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
Methyl Glyoxal MEGLYOX Explicit 3 0 37 5 3.35 4.67 -28% 2.54 2.49 3.57 3.59 3.83<br />
Crotonaldehyde CROTALD PROPALD 10 0 27 1.53 1.71 -11% 1.70<br />
Acetone A<strong>CE</strong>TONE Explicit 4 1 24,39 12,13 0.12 0.18 -33% 0.13 0.14 0.11 0.11 0.11<br />
Methyl Ethyl Ketone MEK Explicit 2 1 37 31 0.35 0.37 -6% 0.49 0.60 0.33 0.37 0.40<br />
C5 Ketones KET5 MEK 10 0 27,32 0.29 0.31 -6% 0.41<br />
C6 Ketones KET6 MEK 10 0 27,32 0.25 0.27 -6% 0.36<br />
Methyl Isobutyl Ketone MIBK Explicit 3 1 30,31,34 31 0.85 1.02 1.19 0.84 0.93 0.98<br />
C7 Ketones KET7 MEK 10 0 27,32 0.22 0.24 -6% 0.31<br />
C8 Ketones KET8 MEK 10 0 27,32 0.20 0.21 -6% 0.28<br />
C9 Ketones KET9 MEK 10 0 27,32 0.18 0.19 -6% 0.25<br />
C10 Ketones KET10 MEK 10 0 27,32 0.16 0.17 -7% 0.23<br />
C5 Cyclic Ketones KET5C MEK 10 0 27,32 0.30 0.32 -6% 0.42<br />
C6 Cyclic Ketones KET6C MEK 10 0 27,32 0.26 0.27 -6% 0.36<br />
C7 Cyclic Ketones KET7C MEK 10 0 27,32 0.23 0.24 -6% 0.32<br />
C8 Cyclic Ketones KET8C MEK 10 0 27,32 0.20 0.21 -6% 0.28<br />
C9 Cyclic Ketones KET9C MEK 10 0 27,32 0.18 0.19 -6% 0.25<br />
C10 Cyclic Ketones KET10C MEK 10 0 27,32 0.16 0.17 -7% 0.23<br />
Diacetone Alcohol DIACTALC Explicit 6 0g 29 31 0.15 0.26 0.30 0.14 0.18 0.18<br />
Methylvinyl ketone MVK Explicit 4 3 13 32 1.68 1.67 1.98 1.71 1.75 1.94<br />
Biacetyl BIA<strong>CE</strong>TYL Explicit 3 5 37 4.31 3.40 3.50 4.59 4.75 5.26<br />
Benzaldehyde BENZALD Explicit 4 3 37,41 5,35 -0.04 -0.18 -76% -1.00 -3.11 -0.08 -0.55 -2.09<br />
Tolualdehyde TOLUALD BENZALD 5 0 8 -0.04 -0.15 -76% -0.88<br />
Phenol PHENOL Explicit 5 0 42 5 0.34 0.36 -5% -0.32 -1.63 0.32 0.07 -0.90<br />
Alkyl Phenols CRESOL Explicit 5 c 42 0.62 0.73 -15% -0.44 -2.49 0.61 0.18 -1.39<br />
o-Cresol O-CRESOL CRESOL 5 5 43 5,10 0.62 -0.44<br />
m-Cresol M-CRESOL CRESOL 5 0 42 0.62 -0.44<br />
p-Cresol P-CRESOL CRESOL 5 0 42 0.62 -0.44<br />
Ethyl Amine ET-AMINE Explicit 10 7 27 1.70 1.70 0% 2.17 2.77 1.69 1.89 2.33<br />
Acrylonitrile ACRYLNIT Explicit 10 0 27 0.52 0.62 -17% 0.69 0.85 0.50 0.65 0.81<br />
Trimethyl Amine TM-AMINE Explicit 10 7 27 1.92 2.43 -21% 1.66 1.73 2.01 2.04 2.27<br />
N-Methyl-2-Pyrrolidone NMP Explicit 4e 1 24 20 0.57 0.91 1.02 0.54 0.65 0.70<br />
Nitrobenzene NO2-BENZ Explicit 10 0 27 0.02 0.02 0.01 0.02 0.02 0.01<br />
Methyl Nitrite ME-NITRT Explicit 3d 0 37 1.93 2.98 -35% 2.01 3.70 2.13 2.72 4.88<br />
Methyl Chloride S-043801 Explicit 10 0 27 0.01 0.01 -4% 0.02 0.02 0.01 0.01 0.01<br />
Dichloromethane S-043802 Explicit 10 0 27 0.01 0.02 -4% 0.03 0.04 0.01 0.02 0.02<br />
Methyl Bromide S-243819 Explicit 10 0 27 0.00 0.00 -4% 0.01 0.01 0.00 0.00 0.01<br />
Chloroform CHCL3 Explicit 10 0 27 0.01 0.01 0.02 0.01 0.01 0.01<br />
Page 9 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
Carbon Tetrachloride CCL4 Inert 0 0 0 0.00 0.00<br />
Methylene Bromide S-043805 Explicit 10 0 27 0.01 0.01 -4% 0.01 0.02 0.01 0.01 0.01<br />
Ethyl Chloride S-043812 Explicit 10 0 27 0.05 0.05 -3% 0.10 0.13 0.05 0.07 0.08<br />
Trans-1,2-Dichloroethene S-099018 Explicit 10 0 27 0.15 0.15 -1% 0.26 0.34 0.13 0.18 0.23<br />
1,1-Dichloroethane S-043813 Explicit 10 0 27 0.02 0.02 -4% 0.04 0.06 0.02 0.03 0.04<br />
1,1,2-Trichloroethane S-043820 Explicit 10 0 27 0.02 0.02 -4% 0.04 0.05 0.02 0.03 0.03<br />
1,1,1-Trichloroethane S-043814 Explicit 10 0 27 0.00 0.00 -4% 0.00 0.00 0.00 0.00 0.00<br />
1,2-Dichloropropane S-099016 Explicit 10 0 27 0.07 0.07 -2% 0.13 0.17 0.07 0.09 0.11<br />
1-Chlorobutane S-098104 Explicit 10 0 27 0.24 0.25 -2% 0.44 0.55 0.22 0.32 0.37<br />
3-(Chloromethyl)-Heptane S-098105 Explicit 10 0 27 0.24 0.24 -1% 0.44 0.49 0.22 0.33 0.34<br />
n-Propyl Bromide C3-BR Explicit 5 1 24,44 25 0.24 0.00 -0.35 0.21 0.08 -0.14<br />
n-Butyl Bromide C4-BR Explicit 5e 1 24,44 25 0.35 0.16 -0.19 0.31 0.20 -0.05<br />
Vinyl Chloride CL-ETHE Explicit 10 0 27 0.63 1.00 1.28 0.60 0.76 0.93<br />
1,1-Dichloroethene 11CL2ETH Explicit 10 0 27 0.47 0.72 0.91 0.44 0.55 0.68<br />
Ethylene Dichloride S-043815 Explicit 10 0 27 0.18 0.18 -2% 0.31 0.40 0.16 0.22 0.27<br />
Trichloroethylene CL3-ETHE Explicit 4c 1 24,45 21 0.24 0.30 0.34 0.22 0.25 0.29<br />
Perchloroethylene CL4-ETHE Explicit 10 0 27 0.01 0.02 0.02 0.01 0.01 0.01<br />
Ethylene Dibromide S-099014 Explicit 10 0 27 0.09 0.09 -2% 0.17 0.21 0.09 0.12 0.14<br />
2-(Cl-methyl)-3-Cl-Propene CL2IBUTE Explicit 9c 2 27 12 0.56 0.56 0.64 0.58 0.63 0.75<br />
Monochlorobenzene CL-BEN Explicit 10 0 27 0.08 0.07 22% 0.07 0.03 0.08 0.08 0.04<br />
p-Dichlorobenzene CL2-BEN Explicit 10 0 27 0.03 0.02 74% 0.03 0.01 0.03 0.03 0.01<br />
p-Trifluoromethyl-Cl-Benzene PCBTF Explicit 7 0 46 37 0.04 0.03 0.01 0.04 0.03 0.02<br />
Benzotrifluoride CF3-BEN Explicit 7 0 46 37 0.09 0.07 0.02 0.08 0.07 0.04<br />
Chloropicrin CCL3NO2 Explicit 3 1 24 29 0.26 0.44 1.12 0.28 0.37 0.84<br />
[a] Species name as used in model.<br />
[b] Representation in model: "Explicit" = represented explicitly; DMSname = represented by indicated species; (blank) = not represented.<br />
[c] See Tables 2-4 for comments or references, where: Unc = uncertainty notes given in Table 2; Exp = notes concerning experimental data for mechanism<br />
evaluation given in Table 3; Notes = documentation notes and comments given in Table 5; Refs = references given in Table 5.<br />
[d] MIR = maximum incremental reactivity, in grams O3 per gram <strong>VOC</strong> emitted, relative to the maximum incremental reactivity of the average of all reactive organic<br />
gas (ROG) emissions, with ozone quantified by peak 1-day ozone yield. "1991" is from the MIR scale calculated in 1991 [6] and incorporated in the CARB Clean<br />
Fuel/Low Emissions Vehicle regulations [4]. "1997" is the PRELIMINARY updated MIR scale calculated using the base mechanism documented by Carter et al<br />
(1997) [22]. Note that the "1997" scale was calculated using the single "averaged conditions" MIR scenario, while the "1991" scale is the average of reactivities<br />
for the 39 MIR scenarios representing different urban areas. The effect of this difference in methodology is expected to be small compared to the effects of the<br />
mechanism updates.<br />
[e] The absolute MIRs of the base ROG mixture are 4.06 gm O3 per gm <strong>VOC</strong> in the 1997 scale and 3.16 gm O3 per gm <strong>VOC</strong> in the 1991 scale. These can be used<br />
to obtain absolute MIRs for the tabulated <strong>VOC</strong>s.<br />
Page 10 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Compound or Mixture DMSname Represent. Notes and References [c] Reactivity Relative to Base ROG (gram basis) [d]<br />
MIR [e]<br />
Other 1997 Scales [f]<br />
[a] [b] Unc Exp Notes Refs 1997 1991 ∆% MOIR EBIR MIR-I MOIR-I EBIR-I<br />
[f]<br />
MOIR and EBIR = maximum ozone incremental reactivity and equal benefit incremental reactivity, respectively, calculated on the same basis as the MIR scale<br />
discussed in Footnote [d]. MIR-I, MOIR-I, and EBIR-I are the incremental reactivities calculated for the MIR, MOIR, or EBIR scales with ozone quantified by<br />
integrated ozone over 0.12 ppm, also relative to the average of all ROG emissions, and calculated on an ozone impact per gram <strong>VOC</strong> emitted basis. These were<br />
calculated using the "averaged conditions" MIR, MOIR, or EBIR scenario.<br />
Page 11 of 11
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-2. Uncertainty notes<br />
Unc.<br />
Note<br />
0 Not applicable: no estimated mechanism, or believed to be unreactive.<br />
1 Considered to be least uncertain<br />
2 Some uncertainties but not likely to change significantly<br />
3 Uncertain but not likely to change significantly<br />
4 Uncertain adjusted mechanism may change somewhat if refined, but change<br />
not expected to be large. If the compound is predicted to inhibit O3, changes<br />
are not expected to affect predicted inhibition, but may affect magnitude of<br />
inhibition.<br />
5 Uncertain and may change if compound is studied (or studied further) or<br />
estimation methods are updated.<br />
6 Uncertain and is expected to change if compound is studied or estimation<br />
methods are updated.<br />
7 Non-negligible chance of estimate being significantly incorrect<br />
8 Current mechanism is expected to (or has been found to) overpredict reactivity.<br />
9 Current mechanism is expected to (or has been found to) underpredict<br />
reactivity.<br />
10 Current mechanism is probably incorrect, but biases in atmospheric reactivity<br />
predictions are uncertain.<br />
a<br />
b<br />
c<br />
d<br />
e<br />
Some uncertainty due to differences in reactivities of compounds represented<br />
by this class. Look at differences among compounds in this class for magnitude<br />
of this uncertainty.<br />
Model does not successfully simulate results of all chamber experiments. This<br />
may be due to experimental difficulties, though mechanism problems cannot be<br />
completely ruled out.<br />
Model does not successfully simulate results of some chamber experiments.<br />
The reactivity of this compound may be unusually sensitive to changes in the<br />
base mechanism.<br />
Model does not fit data well under low NOx conditions, but appears to perform<br />
reasonably well under conditions representing MIR.<br />
Page 1 of 8
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-3. Availability of experimental data for mechanism evaluation.<br />
Exp.<br />
Note<br />
0 No data available to test ozone predictions for this compound.<br />
1 Tested under MIR and other conditions; well tested.<br />
2 Tested under MIR conditions<br />
3 Tested under some conditions, but not MIR reactivity<br />
4 Tested under MIR conditions, but data limited, or of low quality or precision.<br />
5 Tested under some conditions, but data limited, of low precision, or not well<br />
characterized<br />
6 Some qualitative experimental data not consistent with mechanism<br />
7 Data may be available, but mechanism not tested.<br />
a<br />
b<br />
c<br />
d<br />
e<br />
f<br />
g<br />
h<br />
Data available for complex mixtures containing significant amounts of<br />
compounds of this class.<br />
Data not completely processed, but some preliminary modeling carried out.<br />
Data not used in development of current mechanism.<br />
Experimental data available for some members of this class.<br />
Tested under MIR and other conditions, but not as comprehensively as the other<br />
aromatics with experimental data.<br />
A variety of single aromatic - NOx experiments were carried out to evaluate the<br />
mechanism using two different light sources.<br />
Data available only for blacklight chamber. Effect of changing light source is<br />
uncertain and needs to be evaluated.<br />
Attempts to conduct environmental chamber experiments with this compound<br />
have been unsuccessful because of experimental difficulties.<br />
The effect of changing the base ROG surrogate composition has not been<br />
examined. Effect of base ROG composition is uncertain and needs to be<br />
evaluated.<br />
Page 2 of 8
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-4. Documentation notes and comments.<br />
Note.<br />
Documentation or Comment [a]<br />
0 No mechanism assigned. Expected to be of low or negligible reactivity.<br />
1 Mechanism believed to be fairly well established. See Atkinson reviews [1-3].<br />
2 Mechanism estimated using general estimation method for alkanes discussed<br />
by Carter and Atkinson [8] as updated by Atkinson [3].<br />
Consistent with data for normal alkanes only if nitrate formation from hydroxyalkoxy<br />
radicals formed following isomerization is assumed to be much less<br />
important than expected.<br />
3 Estimated mechanism may slightly overpredict reactivities for C14+ n-alkanes.<br />
No data to verify validity of extrapolation above C16 n-alkanes.<br />
4 General alkane estimation method has been found to perform poorly in<br />
simulating reactivities of branched alkanes. Future research may result in<br />
improved estimation methods for branched alkanes.<br />
5 The applicability of the general alkane estimation methods to this compound is<br />
uncertain.<br />
6 The general alkane mechanism appears to perform reasonably well in<br />
simulating reactivity of cyclohexane. However, data with mineral spirits<br />
mixtures and hexyl cyclohexane indicates that it overpredicts reactivities of<br />
higher cycloalkanes.<br />
7 Uncertain whether the compound used to represent this class is most<br />
appropriate for all complex mixtures containing this class.<br />
8 Mechanism estimated by extrapolating from lower molecular weight analogue.<br />
9 Some nitrate formation is assumed to occur in OH reaction for model to<br />
simulate chamber data for 1-hexene [9b]. Nitrate yields for other compounds<br />
estimated based on yields for alkanes.<br />
10 Organic nitrate yield in OH reaction had to be adjusted upwards to obtain<br />
acceptable fit of model to chamber data. Adjusted yield somewhat higher than<br />
expected.<br />
11 Estimated mechanism based on that for isobutene or 2-butene.<br />
12 Highly approximate mechanism based on those for monoalkenes. Probably not<br />
well suited for these compounds.<br />
13 Based on detailed mechanism of Carter and Atkinson [32]. Isoprene<br />
mechanism used is condensed "one product" version given by Carter [33].<br />
14 Estimated mechanism based on that for acyclic internal alkenes, with<br />
appropriate modifications of estimated products formed.<br />
15 Nitrate yields for these terpenes adjusted to fit results of chamber runs.<br />
16 Details of the mechanism and reaction products are unknown. A parameterized<br />
mechanism was adjusted to fit the chamber data. Current version of the<br />
benzene and alkylbenzene mechanism is given by Carter et al (1997) [22].<br />
17 Assumed to have same mechanism as ethyl benzene, but with kOH for the<br />
specific compound.<br />
18 Assumed to have same mechanism and rate constant as m-xylene (for<br />
dialkylbenzenes) or 1,3,5-trimethylbenzene (for polyalkylbenzenes). This may<br />
overestimate reactivities of some isomers.<br />
19 Details of the mechanism and reaction products are unknown. A parameterized<br />
mechanism was adjusted to fit the limited chamber data base as discussed by<br />
Carter et al (1984) [9a]. It has not been updated to be consistent with the<br />
current base mechanism.<br />
20 Assumed to have the same mechanism and rate constant as tetralin.<br />
Page 3 of 8
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-4. Documentation notes and comments.<br />
Note.<br />
Documentation or Comment [a]<br />
21 Interpolated mechanism based on the optimized mechanisms for naphthalene<br />
and 2,3-dimethyl naphthalene.<br />
22 Assumed to have same mechanism and rate constant as 2,3-dimethyl<br />
naphthalene.<br />
23 Estimated mechanism based on assuming most of reaction with OH is at double<br />
bond, reacting in a manner analogous to alkenes.<br />
24 An experimental and modeling study of the reactivity of this compound has<br />
recently been completed. See reference(s) cited.<br />
25 The base mechanism needs to be updated before the reactivity of this<br />
compound will be predicted correctly.<br />
26 The current mechanism for glyoxal, the main product of acetylene, results in<br />
significant underpredictions of the reactivity of acetylene. The mechanism has<br />
not yet been updated to take this into account.<br />
27 A highly approximate "placeholder" mechanism is used so this compound can<br />
be represented in model simulations where this is present in complex mixtures.<br />
28 Mechanism estimated using available estimation methods as of 1991-1993.<br />
Estimates not reviewed or updated recently.<br />
29 Mechanism estimated using automated procedure based on the estimation<br />
methods developed by Atkinson (1997) [3] for alkanes and alkenes. Estimates<br />
generalized to apply to oxygenated compounds.<br />
Note that this method tends to overestimate the relative importances of alkoxy<br />
radical isomerizations and underestimate decompositions. Therefore, estimated<br />
mechanisms may be incorrect in many cases.<br />
Note also that alkyl nitrate yields estimated by this method are uncertainty. This<br />
is important in reactivity predictions for higher molecular weight compounds.<br />
30 This compound is being studied as part of the ARB-funded project on the<br />
reactivity of consumer product <strong>VOC</strong>s.<br />
31 The mechanism for this compound is being updated for an ongoing project. The<br />
reactivities and uncertainty assignments given are for the current mechanism,<br />
which has not been updated to incorporate the new data.<br />
32 It is expected that the estimated mechanism will be updated when work on<br />
related compounds is completed.<br />
33 See Carter et al (1993) [12] for a discussion for the mechanism and data for this<br />
compound.<br />
34 An updated version of this mechanism which is consistent with available<br />
chamber and laboratory data has been incorporated in the model.<br />
35 An updated version of this mechanism which takes into account some of the<br />
new chamber data and/or mechanism estimates has been incorporated, but the<br />
mechanism is still being refined as part of an ongoing project.<br />
36 An adjusted parameterized mechanism has been developed based on chamber<br />
data from a previous study (Carter et al, 1984) [9a], but the current base<br />
mechanism does not incorporate the product species necessary to represent this<br />
appropriately.<br />
37 Most of mechanism is believed to be fairly well established, but there are<br />
uncertainties in the photolysis rates or mechanism which may affect reactivity<br />
predictions.<br />
Page 4 of 8
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-4. Documentation notes and comments.<br />
Note.<br />
Documentation or Comment [a]<br />
38 Several alternative mechanisms were developed as part of a paper study on the<br />
potential ranges of reactivity of this compound (Atkinson and Carter [36]), but no<br />
preferred version was implemented in the model.<br />
39 The mechanism which best fits the chamber data uses an adjustment to the<br />
quantum yields which was not incorporated in the standard mechanism.<br />
Therefore, this mechanism may slightly overestimate the reactivity of acetone.<br />
See Carter et al (1993) [13].<br />
40 An estimated mechanism was developed and adjusted by Carter et al (1984)<br />
[9b], and was subsequently updated. However, some aspects may be out of<br />
date.<br />
41 Photolysis rates were adjusted based on simulations of early chamber<br />
experiments during development of 1990 mechanism, and have not been reevaluated.<br />
Re-adjustment not expected to affect reactivity predictions.<br />
42 Mechanism based on the empirical mechanism derived for o-cresol. Cresol<br />
isomers assumed to have the same mechanism as o-cresol. Phenol<br />
mechanism is derived by analogy.<br />
43 Empirical, highly parameterized and over-simplified mechanism was adjusted to<br />
fit results of a limited number of cresol - NOx chamber runs [5,10].<br />
44 Results of the chamber experiments could not be simulated unless some<br />
unknown process, represented by reaction of O3 with HBr forming radical<br />
initiating species, is assumed. The role of surface reactions in affecting the<br />
chamber results is uncertain.<br />
45 Some process in the T<strong>CE</strong> photooxidation is not adequately represented in the<br />
mechanism. Mechanism might somewhat underestimate MIR reactivities but is<br />
expected to significantly overestimate MOIR and low NOx reactivities.<br />
46 Mechanism estimated based on the mechanism for toluene, with 20% Cl<br />
formation from PCBTF, as discussed in a consulting report [37].<br />
47 A parameterized mechanism was adjusted to fit mini-surrogate reactivity data<br />
with varying NOx. See Carter et al (1992) [11]. The siloxane reactions have<br />
not been incorporated into the current model.<br />
[a] Numbers in brackets refer to reference number on Table 5.<br />
Page 5 of 8
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-5. References<br />
Ref.<br />
Citation<br />
1 Atkinson, R. (1990): "Gas-Phase Tropospheric Chemistry of Organic<br />
Compounds: A Review," Atmos. Environ., 24A, 1-24.<br />
2 Atkinson, R. (1994): "Gas-Phase Tropospheric Chemistry of Organic<br />
Compounds," J. Phys. Chem. Ref. Data, Monograph No. 2.<br />
3 Atkinson, R. (1997): "Gas Phase Tropospheric Chemistry of Volatile Organic<br />
Compounds: 1. Alkanes and Alkenes," J. Phys. Chem. Ref. Data, 26, 215-290.<br />
4 CARB (1993): "Proposed Regulations for Low-Emission Vehicles and Clean<br />
Fuels -- Staff Report and Technical Support Document," California Air<br />
Resources Board, Sacramento, CA, August 13, 1990.<br />
See also Appendix VIII of "California Exhaust Emission Standards and Test<br />
Procedures for 1988 and Subsequent Model Passenger Cars, Light Duty Trucks<br />
and Medium Duty Vehicles," as last amended September 22, 1993.<br />
Incorporated by reference in Section 1960.<br />
5 Carter, W. P. L. (1990): "A Detailed Mechanism for the Gas-Phase Atmospheric<br />
Reactions of Organic Compounds," Atmos. Environ., 24A, 481-518.<br />
6 Carter, W. P. L. (1994): "Development of Ozone Reactivity Scales for Volatile<br />
Organic Compounds," J. Air & Waste Manage. Assoc., 44, 881-899.<br />
7 Carter, W. P. L. (1995): "Computer Modeling of Environmental Chamber<br />
Measurements of Maximum Incremental Reactivities of Volatile Organic<br />
Compounds," Atmos. Environ., 29, 2513-2517.<br />
8 Carter, W. P. L., and R. Atkinson (1985): "Atmospheric Chemistry of Alkanes",<br />
J. Atmos. Chem., 3, 377-405, 1985.<br />
9a Carter, W. P. L., Winer, A. M., Atkinson, R., Dodd, M. C. and Aschmann, S. A.<br />
(1984b): Atmospheric Photochemical Modeling of Turbine Engine Fuels. Phase<br />
I. Experimental studies. Final Report to the USAF, ESL-TR-84-32, September.<br />
9b Carter, W. P. L., A. M. Winer, R. Atkinson, S. E. Heffron, M. P. Poe, and M. A.<br />
Goodman (1987): "Atmospheric Photochemical Modeling of Turbine Engine<br />
Fuels. Phase II. Computer Model Development," Report on USAF Contract no.<br />
F08635-83-0278.<br />
10 Carter, W. P. L. and F. W. Lurmann (1991): "Evaluation of a Detailed Gas-<br />
Phase Atmospheric Reaction Mechanism using Environmental Chamber Data,"<br />
Atm. Environ. 25A, 2771-2806.<br />
11 Carter, W. P. L., J. A. Pierce, I. L. Malkina, and D. Luo (1992): "Investigation of<br />
the Ozone Formation Potential of Selected Volatile Silicone Compounds," Final<br />
Report to Dow Corning Corporation, Midland, MI, November.<br />
12 Carter, W. P. L., J. A. Pierce, I. L. Malkina, D. Luo and W. D. Long (1993):<br />
"Environmental Chamber Studies of Maximum Incremental Reactivities of<br />
Volatile Organic Compounds," Report to Coordinating Research Council, Project<br />
No. ME-9, CARB, etc. [a]<br />
13 Carter, W. P. L, D. Luo, I. L. Malkina, and J. A. Pierce (1993): "An Experimental<br />
and Modeling Study of the Photochemical Ozone Reactivity of Acetone," Final<br />
Report to Chemical Manufacturers Association Contract No. KET-A<strong>CE</strong>-CRC-2.0.<br />
December 10. [a]<br />
14 Carter, W. P. L., J. A. Pierce, D. Luo, and I. L. Malkina (1995): "Environmental<br />
Chamber Studies of Maximum Incremental Reactivities of Volatile Organic<br />
Compounds," Atmos. Environ. 29, 2499-2511.<br />
Page 6 of 8
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-5. References<br />
Ref.<br />
Citation<br />
15 Carter, W. P. L., D. Luo, I. L. Malkina, and J. A. Pierce (1995): "Environmental<br />
Chamber Studies of Atmospheric Reactivities of Volatile Organic Compounds.<br />
Effects of Varying ROG Surrogate and NOx," Report to Coordinating Research<br />
Council, CARB, etc [a]<br />
16 Carter, W. P. L., D. Luo, I. L. Malkina, and J. A. Pierce (1995): "Environmental<br />
Chamber Studies of Atmospheric Reactivities of Volatile Organic Compounds.<br />
Effects of Varying Chamber and Light Source," Final report to NREL, CRC,<br />
CARB, etc. [a]<br />
17 Carter, W. P. L., D. Luo, I. L. Malkina, and D. Fitz (1995): "The University of<br />
California, Riverside Environmental Chamber Data Base for Evaluating Oxidant<br />
Mechanism. Indoor Chamber Experiments through 1993," Report submitted to<br />
the U. S. EPA [a]<br />
18 Carter, W. P. L., D. Luo, and I. L. Malkina (1996): "Investigation of Atmospheric<br />
Ozone Formation Potentials of C12 - C16 n-Alkanes," Report to the Aluminum<br />
Association, October 28. [a]<br />
19 Carter, W. P. L., D. Luo, and I. L. Malkina (1996): "Investigation of the<br />
Atmospheric Ozone Impact of Methyl Acetate," Report to Eastman Chemical<br />
Company, July. [a]<br />
20 Carter, W. P. L., D. Luo, and I. L. Malkina (1996): "Investigation of the<br />
Atmospheric Ozone Formation Potential of t-Butyl Alcohol, N-Methyl<br />
Pyrrolidinone and Propylene Carbonate," Report to ARCO Chemical<br />
Corporation, July 8. [a]<br />
21 Carter, W. P. L., D. Luo, and I. L. Malkina (1996): "Investigation of the<br />
Atmospheric Ozone Formation Potential of Trichloroethylene," Report to the<br />
Halogenated Solvents Industry Alliance, August. [a]<br />
22 Carter, W. P. L., D. Luo, and I. L. Malkina (1997): "Environmental Chamber<br />
Studies for Development of an Updated Photochemical Mechanism for <strong>VOC</strong><br />
Reactivity Assessment," Draft final report to CARB, CRC, NREL [a]<br />
23 Carter, W. P. L., D. Luo, and I. L. Malkina (1997): "Investigation of the<br />
Atmospheric Ozone Formation Potential of Propylene Glycol," Report to Philip<br />
Morris, USA, May 2. [a]<br />
24 Carter, W. P. L., D. Luo, and I. L. Malkina (1997): "Investigation of the<br />
Atmospheric Ozone Formation Potential of Acetylene," Report to Carbind<br />
Graphite Corp., April 1. [a]<br />
25 Carter, W. P. L., D. Luo, and I. L. Malkina (1997): "Investigation of the<br />
Atmospheric Ozone Formation Potential of Selected Alkyl Bromides," Report to<br />
Albemarle Corporation, November 10. [a]<br />
26 Carter, W. P. L., D. Luo, and I. L. Malkina (1997): "Investigation of the<br />
Atmospheric Ozone Formation Potentials of Selected Dibasic Esters," Report to<br />
the Dibasic Esters Group, SOCMA, May 15. [a]<br />
27 Carter, W. P. L., D. Luo, and I. L. Malkina (1997): "Investigation of the<br />
Atmospheric Ozone Formation Potentials of Selected Mineral Spirits Mixtures,"<br />
Report to Safety-Kleen Corporation, July 25. [a]<br />
28 Carter, W. P. L., D. Luo, and I. L. Malkina (1997): "Investigation of the<br />
Atmospheric Ozone Formation Potential of t-Butyl Acetate," Report to ARCO<br />
Chemical Corporation, July 2. [a]<br />
Page 7 of 8
<strong>SUMMARY</strong> <strong>OF</strong> <strong>STATUS</strong> <strong>OF</strong> <strong>VOC</strong> <strong>REACTIVITY</strong> ESTIMATES AS <strong>OF</strong> 3/6/98<br />
Table A-5. References<br />
Ref.<br />
Citation<br />
29 Carter, W. P. L., D. Luo and I. L. Malkina (1997): "Investigation of that<br />
Atmospheric Reactions of Chloropicrin," Atmos. Environ. 31, 1425-1439.; Report<br />
to the Chloropicrin Manufacturers Task Group, May 19. [a]<br />
30 Kwok, E. S. C., and R. Atkinson (1995): "Estimation of Hydroxyl Radical<br />
Reaction Rate Constants for Gas-Phase Organic Compounds Using a Structure-<br />
Reactivity Relationship: An Update," Atmos. Environ 29, 1685-1695.<br />
31 Carter, W. P. L., unpublished results.<br />
32 Carter, W. P. L. and R. Atkinson (1996): "Development and Evaluation of a<br />
Detailed Mechanism for the Atmospheric Reactions of Isoprene and NOx," Int. J.<br />
Chem. Kinet., 28, 497-530.<br />
33 Carter, W. P. L. (1996): "Condensed Atmospheric Photooxidation Mechanisms<br />
for Isoprene," Atmos. Environ., 30, 4275-4290.<br />
34 Carter, W. P. L. (1987): "An Experimental and Modeling Study of the<br />
Photochemical Reactivity of Heatset Printing Oils," Report #2 on U. S. EPA<br />
Cooperative Agreement No. CR810214-01.<br />
35 Carter, W. P. L., R. Atkinson, A. M. Winer, and J. N. Pitts, Jr. (1982):<br />
"Experimental Investigation of Chamber-Dependent Radical Sources," Int. J.<br />
Chem. Kinet., 14, 1071.<br />
36 Atkinson, R., and W. P. L. Carter (1995): "Measurement of OH Radical Reaction<br />
Rate Constants for Purasolv ELS and Purasolv ML and Calculation of Ozone<br />
Formation Potentials," Final Report to Purac America, Inc., June.<br />
37 Carter, W. P. L. Estimation of Atmospheric Reactivity Ranges for<br />
Parachlorobenzotrifluoride and Benzotriflouride," Draft report to Occidental<br />
Chemical Corporation, March, 1998.<br />
[a] This report can be downloaded from the internet at<br />
http://cert.ucr.edu/~carter/bycarter.htm.<br />
Page 8 of 8