You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
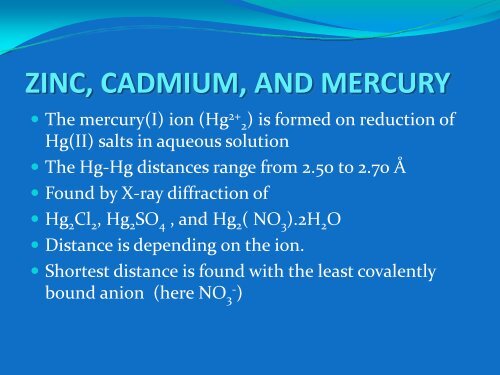
<strong>ZINC</strong>, <strong>CADMIUM</strong>, <strong>AND</strong> <strong>MERCURY</strong><br />
• The mercury(I) ion (Hg 2+ 2) is formed on reduction of<br />
Hg(II) salts in aqueous solution<br />
• The Hg‐Hg distances range from 2.50 to 2.70 Å<br />
• Found by X‐ray diffraction of<br />
• Hg 2 Cl 2 , Hg 2 SO 4 , and Hg 2 ( NO 3 ).2H 2 O<br />
• Distance is depending on the ion.<br />
• Shortest distance is found with the least covalently<br />
bound anion (here NO 3‐ )