Alligator Bioscience AB - Redeye
Alligator Bioscience AB - Redeye
Alligator Bioscience AB - Redeye
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
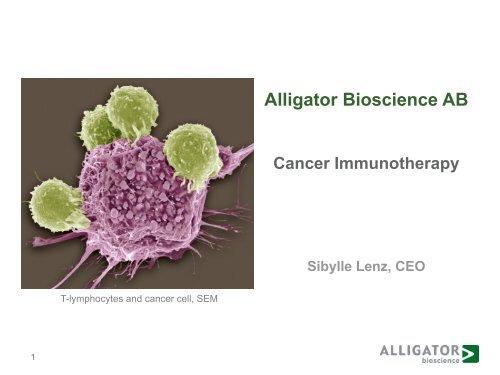
<strong>Alligator</strong> <strong>Bioscience</strong> <strong>AB</strong><br />
Cancer Immunotherapy<br />
Sibylle Lenz, CEO<br />
T-lymphocytes and cancer cell, SEM<br />
1<br />
CONFIDENTIAL
<strong>Alligator</strong> <strong>Bioscience</strong> - 2013<br />
Ø Focus on Cancer Immunotherapy<br />
Ø Growing pipeline of proprietary drug candidates<br />
Ø Novel concept for development of next generation IMmunotherapy<br />
of CANcer (IMCAN) - local administration<br />
Ø New human Antibody library, SCALA-11<br />
Ø Superior protein optimization technology, FIND ® (Fragment<br />
INduced Diversity) for novel and second generation biologics<br />
Ø Strong IP protection on FIND ® and drug candidates<br />
Background: Founded in 2001, Privately owned, 25+ employees<br />
2<br />
CONFIDENTIAL
Technology & Product Growth<br />
FIND ®<br />
Validation<br />
Pipeline<br />
Development<br />
2007<br />
Oncology &<br />
Immunology<br />
2008<br />
2010<br />
Oncology<br />
Immunotherapy<br />
2011 2012<br />
2013<br />
R&D<br />
Company<br />
2001<br />
New CEO &<br />
Mgt Team<br />
<strong>Alligator</strong><br />
Foundation<br />
Pipeline<br />
Focus<br />
IMCAN<br />
Initiation<br />
Antibody<br />
Library<br />
Bispecific<br />
Compounds<br />
Antibody<br />
Formats<br />
3<br />
CONFIDENTIAL
Pipeline Projects<br />
PROJECT MOLECULE INDICATION RESEARCH PRECLINICAL CLINICAL<br />
ADC-1013 Antibody Immunotherapy - Local<br />
2014<br />
ADC-1015 Bispecific Ab Immunotherapy - Local<br />
ADC-1016 Bispecific Ab Immunotherapy - Syst.<br />
2015<br />
2014<br />
External Undisclosed Various<br />
Pipeline Management<br />
Ø One ADC-project in production at all times<br />
Ø Discovery pool of research projects as backup compounds/projects<br />
4<br />
CONFIDENTIAL
Immunotherapy<br />
of Cancer<br />
5<br />
CONFIDENTIAL
Immunotherapy of Cancer<br />
Objective: To develop immunotherapies to boost the immune system<br />
to eradicate tumors and metastases with minimal systemic toxicity.<br />
Clinical Validation of Immunotherapy<br />
Ø Yervoy® (BMS) approved by FDA for metastatic melanoma<br />
Ø Clinical effect at the cost of severe immune-mediated adverse effects<br />
Drawback of Immunotherapy Addressed by Intratumoral Administration*<br />
Ø Concept of local immunotherapy pioneered by Prof. Tötterman<br />
Ø <strong>Alligator</strong> has collaborated with Prof. Tötterman since 2008 in order to develop<br />
the first therapeutic antibodies for local immunotherapy of cancer<br />
6<br />
*) Malmström et al., Clinical Cancer Research 2010:16 : 3279-87<br />
Pesonen et al., Cancer Research 2012: 72: 1621-31<br />
Castro et al., Cancer Research 2012: 72: 2937-48<br />
CONFIDENTIAL
Local Immunotherapy: Competitive Edge<br />
Local immunotherapy of cancer gives superior systemic anticancer<br />
effect with minimal systemic toxicity.<br />
Metastases<br />
Tumor<br />
= immune activation<br />
Systemic immunotherapy:<br />
Unselective immune activation with<br />
limited efficacy and severe toxicity<br />
Local immunotherapy: Tumorselective<br />
immune activation:<br />
improved efficacy, less toxicity*<br />
7<br />
*) von Euler et al., Journal of Immunotherapy 2008: 31: 377-84<br />
Fransen et al., Clinical Cancer Research 2011: 17: 2270-80<br />
CONFIDENTIAL
ADC-1013<br />
CD40 agonistic antibody<br />
8<br />
CONFIDENTIAL
Designing ADC-1013 for Local Immunotherapy<br />
ADC-1013 is FIND®-optimized for superior properties in the tumor<br />
micro-environment, including modifications for both efficacy and safety.<br />
Optimized for local immunotherapy<br />
Ø Full functionality in tumor<br />
microenvironment<br />
Ø High affinity and potency<br />
Ø Strong tumor retention<br />
9<br />
CONFIDENTIAL
In Vivo Data Requirements<br />
<strong>Alligator</strong> has defined specifications for success including essential<br />
criteria for efficacy as well as safety.<br />
Success-criteria local administration<br />
1. Systemic anti-tumor effect<br />
2. Long term tumor immunity<br />
3. Effective at lower dose compared to systemic administration<br />
4. Superior safety profile compared to systemic administration<br />
10<br />
CONFIDENTIAL
1. Systemic Anti-Tumor Effect<br />
Twin tumor model: Two tumors are implanted under the skin, one<br />
tumor receives immunotherapy treatment.<br />
Success criteria: Response to treatment of both tumors à cure<br />
11<br />
CONFIDENTIAL
1. Systemic Anti-Tumor Effect<br />
Local immunotherapy treatment of one tumor (blue) prevents growth<br />
also of the untreated tumor (green).<br />
Rapid tumor growth is seen of the two tumors in control mice (red).<br />
N = 8 in<br />
each group<br />
12<br />
CONFIDENTIAL
2. Long Term Immunity<br />
Cured mice re-challenged with new tumor and without treatment.<br />
Mice are immune to the tumor (cured).<br />
13<br />
CONFIDENTIAL
2. Long Term Immunity<br />
Re-challenge of cured mice (after 2 months) are resistant to tumor<br />
growth (green). Mice without prior treatment are unprotected (red).<br />
†<br />
N = 8 in<br />
each group<br />
14<br />
CONFIDENTIAL
Transgenic Mice<br />
Transgenic mice - generated by insertion of the gene for a human<br />
target in the mouse DNA. The mouse express the human target on<br />
its immune cells. ADC-1013 is tested in this humanized mouse<br />
model.<br />
Wild type mouse<br />
Transgenic mouse<br />
Generation<br />
0<br />
Generation<br />
1<br />
Generation<br />
XX<br />
Breeding<br />
Test of mouse Ab<br />
Test of human Ab<br />
15<br />
CONFIDENTIAL
Transgenic Mice: Bladder Cancer Model<br />
Bladder cancer model in transgenic mice: ADC-1013 is locally<br />
injected: 7 out of 8 mice are cured by ADC-1013 (black), compared to<br />
1 out of 8 mice in the control group (gray)<br />
CR=7 (8)<br />
CR=1 (8)<br />
16<br />
CR= Complete Responders<br />
CONFIDENTIAL
ADC-1013 Status and Development Plan<br />
ADC-1013 is currently being manufactured for toxicology and clinical<br />
studies. Clinical trial in cancer patients in H1 2014.<br />
Toxicology<br />
C<br />
T<br />
A<br />
Clinical<br />
Phase I<br />
Clinical<br />
Phase Ib<br />
C<br />
T<br />
A<br />
Clinical<br />
Phase II<br />
2013 2014 2015<br />
2016<br />
Q1/2 Q3/4 Q1/2 Q3/4 Q1/2 Q3/4<br />
Q1/2 Q3/4<br />
2017<br />
Q1/2 Q3/4<br />
Manufacturing<br />
BD – Partnering activities<br />
CTA = Clinical Trial Application<br />
17<br />
CONFIDENTIAL
ADC-1015<br />
Bispecific antibody<br />
18<br />
CONFIDENTIAL
ADC-1015: A Bispecific Immune Activator<br />
<strong>Alligator</strong> is developing a bispecific immune activating antibody for local<br />
treatment of metastasizing cancer - ADC-1015.<br />
Ø Superior to combination of two monospecific compounds<br />
Ø Novelty – strong IP can be obtained<br />
19<br />
CONFIDENTIAL
ADC-1015: A Bispecific Immune Activator<br />
<strong>Alligator</strong> is developing a bispecific immune activating antibody for local<br />
treatment of metastasizing cancer - ADC-1015.<br />
Ø Superior to combination of two monospecific compounds<br />
Ø Novelty – strong IP can be obtained<br />
ADC-1014 ADC-1015 Undisclosed<br />
20<br />
CONFIDENTIAL
World Class Key Opinion Leaders<br />
<strong>Alligator</strong> is building a network of international opinion leaders within<br />
key areas for immunotherapy of cancer.<br />
Professor Thomas Tötterman et al.,<br />
Clinical Immunology, Uppsala University<br />
Professor Carl Borrebaeck et al.,<br />
Immunotechnology, Lund University<br />
Professor Jeffrey Weber<br />
Immunotherapy of Cancer, Moffitt Cancer Center, US<br />
Professor Ronald Levy et al.,<br />
Immunotherapy of Cancer, Stanford University, US<br />
21
<strong>Alligator</strong> <strong>Bioscience</strong> <strong>AB</strong><br />
Cancer Immunotherapy<br />
Sibylle Lenz, CEO<br />
T-lymphocytes and cancer cell, SEM<br />
22<br />
CONFIDENTIAL
Financing Needs Rest of 2013<br />
Ø Ongoing emission: 46 MSEK<br />
Ø To be issued: 6 613 595 Units<br />
Ø 1 Unit = 1 share + 1 option<br />
Ø Share price = 7 SEK per share<br />
Ø Purchase of one share grants one option to be purchased<br />
later for 3 SEK<br />
Ø The option must be exercised before 15 th July 2014<br />
More information can be found on <strong>Alligator</strong> web-site:<br />
http://www.alligatorbioscience.se/en/<br />
c-195-12Mars2013,<strong>Alligator</strong><strong>Bioscience</strong><strong>AB</strong>genomförriktadnyemission.aspx<br />
23
Financing – Earlier in 2013<br />
Directed emission – according to AGM 2012 resolution:<br />
Ø 12,1 MSEK raised in a directed share issue<br />
Ø Registered in January 2013<br />
Ø New total number of shares: 24 732 949<br />
Ø Total Share Capital: SEK 9 893 180<br />
24
Share Capital – February 18, 2013<br />
Shareholder <br />
Total Shares % Ownership <br />
SUNSTONE CAPITAL 4 880 860 19,73% <br />
STENA AKTIEBOLAG 2 888 088 11,68% <br />
BANQUE INTERNATIONALE À LUXEMBOURG SA 2 522 154 10,20% <br />
STAFFAN RASJÖ 2 098 864 8,49% <br />
LARS SPÅNBERG Incl. Co. 1 921 429 7,77% <br />
SYDSVENSK ENTREPRENÖRFOND <strong>AB</strong> 1 041 666 4,21% <br />
MIKAEL LÖNN 750 000 3,03% <br />
JOHAN RAPP 694 403 2,81% <br />
MAGNUS CLAESSON Incl. Co. 681 949 2,76% <br />
UNIONEN (SIF) 643 833 2,60% <br />
JOHAN CLAESSON, Incl. Co. 576 034 2,33% <br />
PER-‐OLOF MÅRTENSSON Incl. Co. 425 666 1,72% <br />
KENTH PETERSSON 408 000 1,65% <br />
Others 5 200 003 21,02% <br />
Total Number of Shares 24 732 949 100,00% <br />
25