Chapter 4 The Structure of the Atom.pdf
Chapter 4 The Structure of the Atom.pdf
Chapter 4 The Structure of the Atom.pdf
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>The</strong> <strong>Structure</strong> <strong>of</strong> <strong>the</strong> <strong>Atom</strong><br />
Section 4.1 Early Ideas About<br />
Matter<br />
Section 4.2 Defining <strong>the</strong> <strong>Atom</strong><br />
Section 4.3 How <strong>Atom</strong>s Differ<br />
Section 4.4 Unstable Nuclei and<br />
Radioactive Decay<br />
Click a hyperlink or folder tab to view<br />
<strong>the</strong> corresponding slides.<br />
Exit
Section 4.1 Early Ideas About Matter<br />
• Compare and contrast <strong>the</strong> atomic models <strong>of</strong><br />
Democritus, Aristotle, and Dalton.<br />
• Understand how Dalton's <strong>the</strong>ory explains <strong>the</strong><br />
conservation <strong>of</strong> mass.<br />
<strong>the</strong>ory: an explanation supported by many<br />
experiments; is still subject to new experimental<br />
data, can be modified, and is considered<br />
successful if it can be used to make predictions<br />
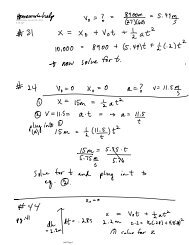
that are true
Section 4.1 Early Ideas About Matter (cont.)<br />
Dalton's atomic <strong>the</strong>ory<br />
<strong>The</strong> ancient Greeks tried to explain<br />
matter, but <strong>the</strong> scientific study <strong>of</strong> <strong>the</strong><br />
atom began with John Dalton in <strong>the</strong><br />
early 1800's.
Greek Philosophers (cont.)<br />
• Many ancient scholars believed matter was<br />
composed <strong>of</strong> such things as earth, water,<br />
air, and fire.<br />
• Many believed<br />
matter could be<br />
endlessly divided<br />
into smaller and<br />
smaller pieces.
Greek Philosophers (cont.)<br />
• Democritus (460–370 B.C.) was <strong>the</strong> first<br />
person to propose <strong>the</strong> idea that matter was<br />
not infinitely divisible, but made up <strong>of</strong><br />
individual particles called atomos.<br />
• Aristotle (484–322 B.C.) disagreed with<br />
Democritus because he did not believe empty<br />
space could exist.<br />
• Aristotle’s views went unchallenged for 2,000<br />
years until science developed methods to test<br />
<strong>the</strong> validity <strong>of</strong> his ideas.
Greek Philosophers (cont.)
Greek Philosophers (cont.)<br />
• John Dalton revived <strong>the</strong> idea <strong>of</strong> <strong>the</strong> atom in<br />
<strong>the</strong> early 1800s based on numerous<br />
chemical reactions.<br />
• Dalton’s atomic <strong>the</strong>ory easily explained<br />
conservation <strong>of</strong> mass in a reaction as <strong>the</strong><br />
result <strong>of</strong> <strong>the</strong> combination, separation, or<br />
rearrangement <strong>of</strong> atoms.
Greek Philosophers (cont.)
Section 4.1 Assessment<br />
Who was <strong>the</strong> first person to propose <strong>the</strong><br />
idea that matter was not infinitely<br />
divisible?<br />
A. Aristotle<br />
B. Plato<br />
C. Dalton<br />
D. Democritus<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Section 4.1 Assessment<br />
Dalton’s <strong>the</strong>ory also conveniently<br />
explained what?<br />
A. <strong>the</strong> electron<br />
B. <strong>the</strong> nucleus<br />
C. law <strong>of</strong> conservation <strong>of</strong> mass<br />
D. law <strong>of</strong> Democritus<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Section 4.2 Defining <strong>the</strong> <strong>Atom</strong><br />
• Define atom.<br />
• Distinguish between <strong>the</strong> subatomic particles in<br />
terms <strong>of</strong> relative charge and mass.<br />
• Describe <strong>the</strong> structure <strong>of</strong> <strong>the</strong> atom, including <strong>the</strong><br />
locations <strong>of</strong> <strong>the</strong> subatomic particles.<br />
model: a visual, verbal, and/or ma<strong>the</strong>matical<br />
explanation <strong>of</strong> data collected from many<br />
experiments
Section 4.2 Defining <strong>the</strong> <strong>Atom</strong> (cont.)<br />
atom<br />
cathode ray<br />
electron<br />
nucleus<br />
proton<br />
neutron<br />
An atom is made <strong>of</strong> a nucleus containing<br />
protons and neutrons; electrons move<br />
around <strong>the</strong> nucleus.
<strong>The</strong> <strong>Atom</strong><br />
• <strong>The</strong> smallest particle <strong>of</strong> an element that<br />
retains <strong>the</strong> properties <strong>of</strong> <strong>the</strong> element is<br />
called an atom.<br />
• An instrument called <strong>the</strong> scanning tunneling<br />
microscope (STM) allows individual atoms to<br />
be seen.
<strong>The</strong> Electron<br />
• When an electric charge is applied, a ray <strong>of</strong><br />
radiation travels from <strong>the</strong> cathode to <strong>the</strong><br />
anode, called a cathode ray.<br />
• Cathode rays are a stream <strong>of</strong> particles<br />
carrying a negative charge.<br />
• <strong>The</strong> particles carrying a negative charge are<br />
known as electrons.
<strong>The</strong> Electron (cont.)<br />
• This figure shows a typical cathode ray<br />
tube.
<strong>The</strong> Electron (cont.)<br />
• J.J. Thomson measured <strong>the</strong> effects <strong>of</strong> both<br />
magnetic and electric fields on <strong>the</strong> cathode<br />
ray to determine <strong>the</strong> charge-to-mass ratio<br />
<strong>of</strong> a charged particle, <strong>the</strong>n compared it to<br />
known values.<br />
• <strong>The</strong> mass <strong>of</strong> <strong>the</strong> charged particle was much<br />
less than a hydrogen atom, <strong>the</strong>n <strong>the</strong> lightest<br />
known atom.<br />
• Thomson received <strong>the</strong> Nobel Prize in 1906<br />
for identifying <strong>the</strong> first subatomic particle—<strong>the</strong><br />
electron
<strong>The</strong> Electron (cont.)<br />
• In <strong>the</strong> early 1910s, Robert Millikan used <strong>the</strong><br />
oil-drop apparatus shown below to<br />
determine <strong>the</strong> charge <strong>of</strong> an electron.
<strong>The</strong> Electron (cont.)<br />
• Charges change in discrete amounts—<br />
1.602 10 –19 coulombs, <strong>the</strong> charge <strong>of</strong> one<br />
electron (now equated to a single unit, 1–).<br />
• With <strong>the</strong> electron’s charge and charge-tomass<br />
ratio known, Millikan calculated <strong>the</strong><br />
mass <strong>of</strong> a single electron.<br />
<strong>the</strong> mass <strong>of</strong><br />
a hydrogen<br />
atom
<strong>The</strong> Electron (cont.)<br />
• Matter is neutral.<br />
• J.J. Thomson's plum pudding model <strong>of</strong> <strong>the</strong><br />
atom states that <strong>the</strong> atom is a uniform,<br />
positively changed sphere containing<br />
electrons.
<strong>The</strong> Nucleus<br />
• In 1911, Ernest Ru<strong>the</strong>rford studied how<br />
positively charged alpha particles<br />
interacted with solid matter.<br />
• By aiming <strong>the</strong> particles at<br />
a thin sheet <strong>of</strong> gold foil,<br />
Ru<strong>the</strong>rford expected <strong>the</strong><br />
paths <strong>of</strong> <strong>the</strong> alpha<br />
particles to be only<br />
slightly altered by a<br />
collision with an electron.
<strong>The</strong> Nucleus (cont.)<br />
• Although most <strong>of</strong> <strong>the</strong> alpha particles went<br />
through <strong>the</strong> gold foil, a few <strong>of</strong> <strong>the</strong>m<br />
bounced back, some at large angles.
<strong>The</strong> Nucleus (cont.)<br />
• Ru<strong>the</strong>rford concluded that atoms are<br />
mostly empty space.<br />
• Almost all <strong>of</strong> <strong>the</strong> atom's positive charge and<br />
almost all <strong>of</strong> its mass is contained in a dense<br />
region in <strong>the</strong> center <strong>of</strong> <strong>the</strong> atom called <strong>the</strong><br />
nucleus.<br />
• Electrons are held within <strong>the</strong> atom by <strong>the</strong>ir<br />
attraction to <strong>the</strong> positively charged nucleus.
<strong>The</strong> Nucleus (cont.)<br />
• <strong>The</strong> repulsive force between <strong>the</strong> positively<br />
charged nucleus and positive alpha<br />
particles caused <strong>the</strong> deflections.
<strong>The</strong> Nucleus (cont.)<br />
• Ru<strong>the</strong>rford refined <strong>the</strong> model to include<br />
positively charged particles in <strong>the</strong> nucleus<br />
called protons.<br />
• James Chadwick received <strong>the</strong> Nobel Prize in<br />
1935 for discovering <strong>the</strong> existence <strong>of</strong><br />
neutrons, neutral particles in <strong>the</strong> nucleus<br />
which accounts for <strong>the</strong> remainder <strong>of</strong> an<br />
atom’s mass.
<strong>The</strong> Nucleus (cont.)<br />
• All atoms are made <strong>of</strong> three<br />
fundamental subatomic<br />
particles: <strong>the</strong> electron, <strong>the</strong><br />
proton, and <strong>the</strong> neutron.<br />
• <strong>Atom</strong>s are spherically<br />
shaped.<br />
• <strong>Atom</strong>s are mostly empty<br />
space, and electrons travel<br />
around <strong>the</strong> nucleus held by<br />
an attraction to <strong>the</strong> positively<br />
charged nucleus.
<strong>The</strong> Nucleus (cont.)<br />
• Scientists have determined that protons<br />
and neutrons are composed <strong>of</strong> subatomic<br />
particles called quarks.
<strong>The</strong> Nucleus (cont.)<br />
• Chemical behavior can be explained by<br />
considering only an atom's electrons.
Section 4.2 Assessment<br />
<strong>Atom</strong>s are mostly ____.<br />
A. positive<br />
B. negative<br />
C. solid spheres<br />
D. empty space<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Section 4.2 Assessment<br />
What are <strong>the</strong> two fundamental subatomic<br />
particles found in <strong>the</strong> nucleus?<br />
A. proton and electron<br />
B. proton and neutron<br />
C. neutron and electron<br />
D. neutron and positron<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Section 4.3 How <strong>Atom</strong>s Differ<br />
• Explain <strong>the</strong> role <strong>of</strong> atomic number in determining <strong>the</strong><br />
identity <strong>of</strong> an atom.<br />
• Define an isotope.<br />
• Explain why atomic masses are not whole numbers.<br />
• Calculate <strong>the</strong> number <strong>of</strong> electrons, protons, and<br />
neutrons in an atom given its mass number and<br />
atomic number.
Section 4.3 How <strong>Atom</strong>s Differ (cont.)<br />
periodic table: a chart that organizes all known<br />
elements into a grid <strong>of</strong> horizontal rows (periods)<br />
and vertical columns (groups or families) arranged<br />
by increasing atomic number<br />
atomic number<br />
isotopes<br />
mass number<br />
atomic mass unit (amu)<br />
atomic mass<br />
<strong>The</strong> number <strong>of</strong> protons and <strong>the</strong> mass<br />
number define <strong>the</strong> type <strong>of</strong> atom.
<strong>Atom</strong>ic Number<br />
• Each element contains a unique positive<br />
charge in <strong>the</strong>ir nucleus.<br />
• <strong>The</strong> number <strong>of</strong> protons in <strong>the</strong> nucleus <strong>of</strong> an<br />
atom identifies <strong>the</strong> element and is known as<br />
<strong>the</strong> element’s atomic number.
Isotopes and Mass Number<br />
• All atoms <strong>of</strong> a particular element have <strong>the</strong><br />
same number <strong>of</strong> protons and electrons but<br />
<strong>the</strong> number <strong>of</strong> neutrons in <strong>the</strong> nucleus can<br />
differ.<br />
• <strong>Atom</strong>s with <strong>the</strong> same number <strong>of</strong> protons but<br />
different numbers <strong>of</strong> neutrons are called<br />
isotopes.
Isotopes and Mass Number (cont.)<br />
• <strong>The</strong> relative abundance <strong>of</strong> each isotope is<br />
usually constant.<br />
• Isotopes containing more neutrons have a<br />
greater mass.<br />
• Isotopes have <strong>the</strong> same chemical behavior.<br />
• <strong>The</strong> mass number is <strong>the</strong> sum <strong>of</strong> <strong>the</strong> protons<br />
and neutrons in <strong>the</strong> nucleus.
Isotopes and Mass Number (cont.)
Mass <strong>of</strong> <strong>Atom</strong>s<br />
• One atomic mass unit (amu) is defined as<br />
1/12 th <strong>the</strong> mass <strong>of</strong> a carbon-12 atom.<br />
• One amu is nearly, but not exactly, equal to<br />
one proton and one neutron.
Mass <strong>of</strong> <strong>Atom</strong>s (cont.)<br />
• <strong>The</strong> atomic mass <strong>of</strong> an element is <strong>the</strong><br />
weighted average mass <strong>of</strong> <strong>the</strong> isotopes <strong>of</strong><br />
that element.
Section 4.3 Assessment<br />
An unknown element has 19 protons, 19<br />
electrons, and 3 isotopes with 20, 21 and<br />
22 neutrons. What is <strong>the</strong> element’s atomic<br />
number?<br />
A. 38<br />
B. 40<br />
C. 19<br />
D. unable to determine<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Section 4.3 Assessment<br />
Elements with <strong>the</strong> same number <strong>of</strong><br />
protons and differing numbers <strong>of</strong><br />
neutrons are known as what?<br />
A. isotopes<br />
B. radioactive<br />
C. abundant<br />
D. ions<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Section 4.4 Unstable Nuclei and<br />
Radioactive Decay<br />
• Explain <strong>the</strong> relationship between unstable nuclei and<br />
radioactive decay.<br />
• Characterize alpha, beta, and gamma radiation in<br />
terms <strong>of</strong> mass and charge.<br />
element: a pure substance that cannot be broken<br />
down into simpler substances by physical or chemical<br />
means
Section 4.4 Unstable Nuclei and<br />
Radioactive Decay (cont.)<br />
radioactivity<br />
radiation<br />
nuclear reaction<br />
radioactive decay<br />
alpha radiation<br />
alpha particle<br />
nuclear equation<br />
beta radiation<br />
beta particle<br />
gamma rays<br />
Unstable atoms emit radiation to gain<br />
stability.
Radioactivity<br />
• Nuclear reactions can change one element<br />
into ano<strong>the</strong>r element.<br />
• In <strong>the</strong> late 1890s, scientists noticed some<br />
substances spontaneously emitted radiation,<br />
a process <strong>the</strong>y called radioactivity.<br />
• <strong>The</strong> rays and particles emitted are called<br />
radiation.<br />
• A reaction that involves a change in an atom's<br />
nucleus is called a nuclear reaction.
Radioactive Decay<br />
• Unstable nuclei lose energy by emitting<br />
radiation in a spontaneous process called<br />
radioactive decay.<br />
• Unstable radioactive elements undergo<br />
radioactive decay thus forming stable<br />
nonradioactive elements.
Radioactive Decay (cont.)<br />
• Alpha radiation is made up <strong>of</strong> positively<br />
charged particles called alpha particles.<br />
• Each alpha particle contains two protons and<br />
two neutrons and has a 2 + charge.
Radioactive Decay (cont.)<br />
• <strong>The</strong> figure shown below is a nuclear<br />
equation showing <strong>the</strong> radioactive decay <strong>of</strong><br />
radium-226 to radon-222.<br />
• <strong>The</strong> mass is conserved in nuclear equations.
Radioactive Decay (cont.)<br />
• Beta radiation is radiation that has a<br />
negative charge and emits beta particles.<br />
• Each beta particle is an electron with a 1–<br />
charge.
Radioactive Decay (cont.)
Radioactive Decay (cont.)<br />
• Gamma rays are high-energy radiation<br />
with no mass and are neutral.<br />
• Gamma rays account for most <strong>of</strong> <strong>the</strong> energy<br />
lost during radioactive decay.
Radioactive Decay (cont.)<br />
• <strong>Atom</strong>s that contain too many or two few<br />
neutrons are unstable and lose energy<br />
through radioactive decay to form a stable<br />
nucleus.<br />
• Few exist in nature—most have already<br />
decayed to stable forms.
Section 4.4 Assessment<br />
A reaction that changes one element into<br />
ano<strong>the</strong>r is called what?<br />
A. chemical reaction<br />
B. beta radiation<br />
C. nuclear reaction<br />
D. physical reaction<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Section 4.4 Assessment<br />
Why are radioactive elements rare in<br />
nature?<br />
A. <strong>The</strong>y do no occur on Earth.<br />
B. Most have already decayed to a<br />
stable form.<br />
C. <strong>The</strong>y take a long time to form.<br />
D. <strong>The</strong>y are too hard to detect.<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Chemistry Online<br />
Study Guide<br />
<strong>Chapter</strong> Assessment<br />
Standardized Test Practice<br />
Image Bank<br />
Concepts in Motion
Key Concepts<br />
Section 4.1 Early Ideas About Matter<br />
• Democritus was <strong>the</strong> first person to propose <strong>the</strong><br />
existence <strong>of</strong> atoms.<br />
• According to Democritus, atoms are solid,<br />
homogeneous, and indivisible.<br />
• Aristotle did not believe in <strong>the</strong> existence <strong>of</strong> atoms.<br />
• John Dalton’s atomic <strong>the</strong>ory is based on numerous<br />
scientific experiments.
Key Concepts<br />
Section 4.2 Defining <strong>the</strong> <strong>Atom</strong><br />
• An atom is <strong>the</strong> smallest particle <strong>of</strong> an element that<br />
maintains <strong>the</strong> properties <strong>of</strong> that element.<br />
• Electrons have a 1– charge, protons have a 1+ charge,<br />
and neutrons have no charge.<br />
• An atom consists mostly <strong>of</strong> empty space surrounding<br />
<strong>the</strong> nucleus.
Key Concepts<br />
Section 4.3 How <strong>Atom</strong>s Differ<br />
• <strong>The</strong> atomic number <strong>of</strong> an atom is given by its<br />
number <strong>of</strong> protons. <strong>The</strong> mass number <strong>of</strong> an atom is<br />
<strong>the</strong> sum <strong>of</strong> its neutrons and protons.<br />
atomic number = number <strong>of</strong> protons = number <strong>of</strong> electrons<br />
mass number = atomic number + number <strong>of</strong> neutrons<br />
• <strong>Atom</strong>s <strong>of</strong> <strong>the</strong> same element with different numbers <strong>of</strong><br />
neutrons are called isotopes.<br />
• <strong>The</strong> atomic mass <strong>of</strong> an element is a weighted average <strong>of</strong><br />
<strong>the</strong> masses <strong>of</strong> all <strong>of</strong> its naturally occurring isotopes.
Key Concepts<br />
Section 4.4 Unstable Nuclei and<br />
Radioactive Decay<br />
• Chemical reactions involve changes in <strong>the</strong> electrons<br />
surrounding an atom. Nuclear reactions involve<br />
changes in <strong>the</strong> nucleus <strong>of</strong> an atom.<br />
• <strong>The</strong>re are three types <strong>of</strong> radiation: alpha (charge <strong>of</strong> 2+),<br />
beta (charge <strong>of</strong> 1–), and gamma (no charge).<br />
• <strong>The</strong> neutron-to-proton ratio <strong>of</strong> an atom’s nucleus<br />
determines its stability.
Whose work led to <strong>the</strong> modern atomic<br />
<strong>the</strong>ory?<br />
A. Dalton<br />
B. Ru<strong>the</strong>rford<br />
C. Einstein<br />
D. Aristotle<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Which particle is not found in <strong>the</strong> nucleus<br />
<strong>of</strong> an atom?<br />
A. neutron<br />
B. proton<br />
C. gamma ray<br />
D. electron<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Two isotopes <strong>of</strong> an unknown element<br />
have <strong>the</strong> same number <strong>of</strong>:<br />
A. protons<br />
B. neutrons<br />
C. electrons<br />
D. both A and C<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Lithium has an atomic mass <strong>of</strong> 6.941 and<br />
two isotopes, one with 6 neutrons and one<br />
with 7 neutrons. Which isotope is more<br />
abundant?<br />
A. 6 Li<br />
B. 7 Li<br />
C. Both isotopes occur equally.<br />
D. unable to determine<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
What happens when an element emits<br />
radioactive particles?<br />
A. It gains energy.<br />
B. It gains neutrons.<br />
C. It loses stability.<br />
D. It loses energy.<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
What is <strong>the</strong> smallest particle <strong>of</strong> an element<br />
that still retains <strong>the</strong> properties <strong>of</strong> that<br />
element?<br />
A. proton<br />
B. atom<br />
C. electron<br />
D. neutron<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
How many neutrons, protons, and<br />
electrons does 124 54Xe have?<br />
A. 124 neutrons, 54 protons, 54 electrons<br />
B. 70 neutrons, 54 protons, 54 electrons<br />
C. 124 neutrons, 70 protons, 54 electrons<br />
D. 70 neutrons, 70 protons, 54 electrons<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
<strong>The</strong> primary factor in determining an<br />
atom's stability is its ratio <strong>of</strong> neutrons<br />
to ____.<br />
A. protons<br />
B. electrons<br />
C. alpha particles<br />
D. isotopes<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
What is <strong>the</strong> densest region <strong>of</strong> an atom?<br />
A. electron cloud<br />
B. nucleus<br />
C. isotopes<br />
D. atomic mass<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Why are electrons attracted to <strong>the</strong> cathode<br />
in a cathode ray tube?<br />
A. <strong>The</strong> cathode is more stable.<br />
B. <strong>The</strong> cathode has a positive charge.<br />
C. <strong>The</strong> cathode has a negative charge.<br />
D. <strong>The</strong> cathode has no charge.<br />
A<br />
B<br />
A. A<br />
B. B<br />
C. C<br />
0% 0% 0% 0%<br />
D. D<br />
C<br />
D
Click on an image to enlarge.
Table 4.3 Properties <strong>of</strong> Subatomic Particles<br />
Figure 4.12 Ru<strong>the</strong>rford's Experiment<br />
Figure 4.14 Features <strong>of</strong> an <strong>Atom</strong><br />
Figure 4.21 Types <strong>of</strong> Radiation
Click any <strong>of</strong> <strong>the</strong> background top tabs<br />
to display <strong>the</strong> respective folder.<br />
Within <strong>the</strong> <strong>Chapter</strong> Outline, clicking a section<br />
tab on <strong>the</strong> right side <strong>of</strong> <strong>the</strong> screen will bring you<br />
to <strong>the</strong> first slide in each respective section.<br />
Simple navigation buttons will allow you to<br />
progress to <strong>the</strong> next slide or <strong>the</strong> previous slide.<br />
<strong>The</strong> <strong>Chapter</strong> Resources Menu will allow you to<br />
access chapter specific resources from <strong>the</strong> <strong>Chapter</strong><br />
Menu or any <strong>Chapter</strong> Outline slide. From within any<br />
feature, click <strong>the</strong> Resources tab to return to this slide.<br />
<strong>The</strong> ―Return‖ button will allow you to return to <strong>the</strong><br />
slide that you were viewing when you clicked ei<strong>the</strong>r<br />
<strong>the</strong> Resources or Help tab.<br />
To exit <strong>the</strong> presentation, click <strong>the</strong> Exit button on <strong>the</strong> <strong>Chapter</strong> Menu slide or hit<br />
Escape [Esc] on your keyboards while viewing any <strong>Chapter</strong> Outline slide.
This slide is intentionally blank.