Instruments for Measurement of Dissolved ... - MEP Instruments
Instruments for Measurement of Dissolved ... - MEP Instruments
Instruments for Measurement of Dissolved ... - MEP Instruments
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
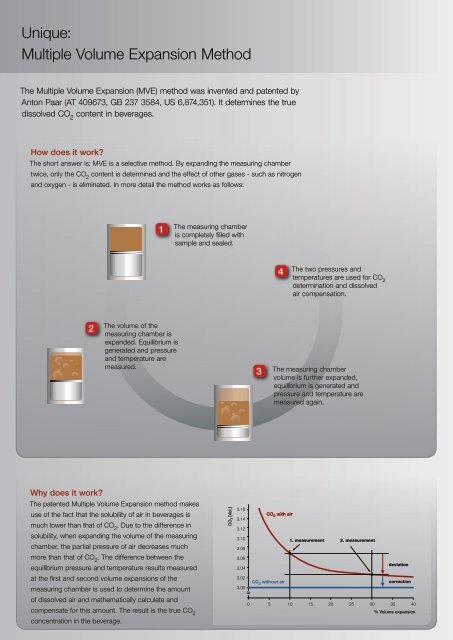
Unique:<br />
Multiple Volume Expansion Method<br />
The Multiple Volume Expansion (MVE) method was invented and patented by<br />
Anton Paar (AT 409673, GB 237 3584, US 6,874,351). It determines the true<br />
dissolved CO 2<br />
content in beverages.<br />
How does it work?<br />
The short answer is: MVE is a selective method. By expanding the measuring chamber<br />
twice, only the CO 2<br />
content is determined and the effect <strong>of</strong> other gases - such as nitrogen<br />
and oxygen - is eliminated. In more detail the method works as follows:<br />
1<br />
The measuring chamber<br />
is completely filled with<br />
sample and sealed.<br />
4<br />
The two pressures and<br />
temperatures are used <strong>for</strong> CO 2<br />
determination and dissolved<br />
air compensation.<br />
2<br />
The volume <strong>of</strong> the<br />
measuring chamber is<br />
expanded. Equilibrium is<br />
generated and pressure<br />
and temperature are<br />
measured.<br />
3<br />
The measuring chamber<br />
volume is further expanded,<br />
equilibrium is generated and<br />
pressure and temperature are<br />
measured again.<br />
Why does it work?<br />
The patented Multiple Volume Expansion method makes<br />
use <strong>of</strong> the fact that the solubility <strong>of</strong> air in beverages is<br />
much lower than that <strong>of</strong> CO 2<br />
. Due to the difference in<br />
solubility, when expanding the volume <strong>of</strong> the measuring<br />
chamber, the partial pressure <strong>of</strong> air decreases much<br />
more than that <strong>of</strong> CO 2<br />
. The difference between the<br />
equilibrium pressure and temperature results measured<br />
at the first and second volume expansions <strong>of</strong> the<br />
measuring chamber is used to determine the amount<br />
<strong>of</strong> dissolved air and mathematically calculate and<br />
compensate <strong>for</strong> this amount. The result is the true CO 2<br />
concentration in the beverage.






![Rice, size measurement of broken grains [pdf] - MEP Instruments](https://img.yumpu.com/46724497/1/184x260/rice-size-measurement-of-broken-grains-pdf-mep-instruments.jpg?quality=85)