Science - Teachers Sri Lanka
Science - Teachers Sri Lanka
Science - Teachers Sri Lanka
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
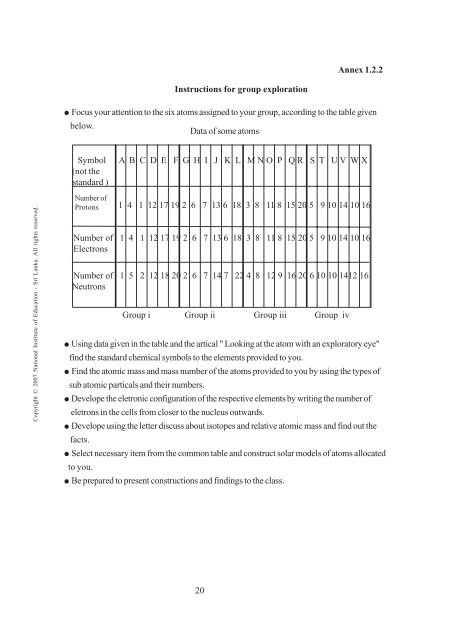
Annex 1.2.2<br />
Instructions for group exploration<br />
² Focus your attention to the six atoms assigned to your group, according to the table given<br />
below.<br />
Data of some atoms<br />
Symbol<br />
(not the<br />
standard )<br />
A B C D E F G H I J K L M N O P Q R S T U V W X<br />
Copyright © 2007 National Institute of Education - <strong>Sri</strong> <strong>Lanka</strong>. All rights reserved.<br />
Number of<br />
Protons<br />
1 4 1 12 17 19 2 6 7 13 6 18 3 8 11 8 15 20 5 9 10 14 10 16<br />
Number of 1 4 1 12 17 19 2 6 7 13 6 18 3 8 11 8 15 20 5 9 10 14 10 16<br />
Electrons<br />
Number of 1 5 2 12 18 20 2 6 7 14 7 22 4 8 12 9 16 20 6 10 10 1412 16<br />
Neutrons<br />
Group i Group ii Group iii Group iv<br />
² Using data given in the table and the artical " Looking at the atom with an exploratory eye"<br />
find the standard chemical symbols to the elements provided to you.<br />
² Find the atomic mass and mass number of the atoms provided to you by using the types of<br />
sub atomic particals and their numbers.<br />
² Develope the eletronic configuration of the respective elements by writing the number of<br />
eletrons in the cells from closer to the nucleus outwards.<br />
² Develope using the letter discuss about isotopes and relative atomic mass and find out the<br />
facts.<br />
² Select necessary item from the common table and construct solar models of atoms allocated<br />
to you.<br />
² Be prepared to present constructions and findings to the class.<br />
20