The Pharmaceutical Price Regulation Scheme - Office of Fair Trading
The Pharmaceutical Price Regulation Scheme - Office of Fair Trading
The Pharmaceutical Price Regulation Scheme - Office of Fair Trading
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
February 2007<br />
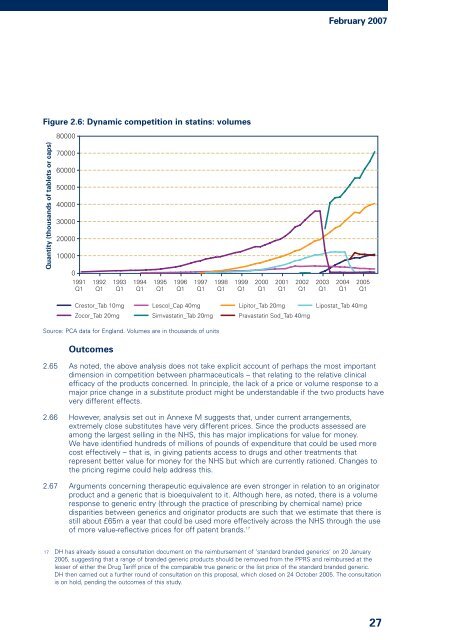
Figure 2.6: Dynamic competition in statins: volumes<br />
80000<br />
Quantity (thousands <strong>of</strong> tablets or caps)<br />
70000<br />
60000<br />
50000<br />
40000<br />
30000<br />
20000<br />
10000<br />
0<br />
1991<br />
Q1<br />
1992<br />
Q1<br />
1993<br />
Q1<br />
1994<br />
Q1<br />
1995<br />
Q1<br />
1996<br />
Q1<br />
1997<br />
Q1<br />
1998<br />
Q1<br />
1999<br />
Q1<br />
2000<br />
Q1<br />
2001<br />
Q1<br />
2002<br />
Q1<br />
2003<br />
Q1<br />
2004<br />
Q1<br />
2005<br />
Q1<br />
Crestor_Tab 10mg<br />
Zocor_Tab 20mg<br />
Lescol_Cap 40mg<br />
Simvastatin_Tab 20mg<br />
Lipostat_Tab 40mg<br />
Lipitor_Tab 20mg<br />
Pravastatin Sod_Tab 40mg<br />
Source: PCA data for England. Volumes are in thousands <strong>of</strong> units<br />
Outcomes<br />
2.65 As noted, the above analysis does not take explicit account <strong>of</strong> perhaps the most important<br />
dimension in competition between pharmaceuticals – that relating to the relative clinical<br />
efficacy <strong>of</strong> the products concerned. In principle, the lack <strong>of</strong> a price or volume response to a<br />
major price change in a substitute product might be understandable if the two products have<br />
very different effects.<br />
2.66 However, analysis set out in Annexe M suggests that, under current arrangements,<br />
extremely close substitutes have very different prices. Since the products assessed are<br />
among the largest selling in the NHS, this has major implications for value for money.<br />
We have identified hundreds <strong>of</strong> millions <strong>of</strong> pounds <strong>of</strong> expenditure that could be used more<br />
cost effectively – that is, in giving patients access to drugs and other treatments that<br />
represent better value for money for the NHS but which are currently rationed. Changes to<br />
the pricing regime could help address this.<br />
2.67 Arguments concerning therapeutic equivalence are even stronger in relation to an originator<br />
product and a generic that is bioequivalent to it. Although here, as noted, there is a volume<br />
response to generic entry (through the practice <strong>of</strong> prescribing by chemical name) price<br />
disparities between generics and originator products are such that we estimate that there is<br />
still about £65m a year that could be used more effectively across the NHS through the use<br />
<strong>of</strong> more value-reflective prices for <strong>of</strong>f patent brands. 17 27<br />
17 DH has already issued a consultation document on the reimbursement <strong>of</strong> ‘standard branded generics’ on 20 January<br />
2005, suggesting that a range <strong>of</strong> branded generic products should be removed from the PPRS and reimbursed at the<br />
lesser <strong>of</strong> either the Drug Tariff price <strong>of</strong> the comparable true generic or the list price <strong>of</strong> the standard branded generic.<br />
DH then carried out a further round <strong>of</strong> consultation on this proposal, which closed on 24 October 2005. <strong>The</strong> consultation<br />
is on hold, pending the outcomes <strong>of</strong> this study.