Vol 7, No 3 - PHA Online University
Vol 7, No 3 - PHA Online University
Vol 7, No 3 - PHA Online University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Advances in<br />
Pulmonary<br />
Hypertension<br />
Official Journal of the Pulmonary Hypertension Association<br />
Autumn 2008<br />
<strong>Vol</strong> 7, <strong>No</strong> 3<br />
Highlights From<br />
Scientific Sessions<br />
of <strong>PHA</strong>’s International<br />
Conference<br />
See description on page 318<br />
CME in This Issue
Table of Contents<br />
Guest Editor for this issue:<br />
Karen Fagan, MD<br />
<strong>University</strong> of South Alabama<br />
College of Medicine<br />
Mobile, Alabama<br />
320 Profiles in Pulmonary<br />
Hypertension:<br />
Vallerie V. McLaughlin, MD<br />
330 Advances in Pulmonary<br />
Hypertension CME Section<br />
332 The Metabolic Syndrome<br />
and Cardiac Function<br />
337 National Heart Lung and Blood<br />
Institute Hopkins Specialized<br />
Center in Clinical Oriented<br />
Research (SCCOR): Molecular<br />
Determinants of Pulmonary<br />
Arterial Hypertension<br />
341 Specialized Center in Clinical<br />
Oriented Research (SCCOR)<br />
Update: Mechanisms and Treatment<br />
of Lung Vascular Disease<br />
in Infants and Children<br />
343 Getting More From Right Heart<br />
Catheterization: A Focus on the<br />
Right Ventricle<br />
346 Animal Models of Human<br />
Severe PAH<br />
351 Self-Assessment Examination<br />
353 Pulmonary Hypertension<br />
Roundtable Discussion<br />
Publisher<br />
Pulmonary Hypertension Association<br />
Michael D. McGoon, MD, Chair of the Board<br />
Rino Aldrighetti, President<br />
Donica Merhazion, Associate Director of Medical Services<br />
<strong>PHA</strong> Office<br />
Pulmonary Hypertension Association<br />
801 Roeder Rd. Suite 400<br />
Silver Spring, MD 20910-4496<br />
301-565-3004, 301-565-3994 (fax)<br />
www.phassociation.org<br />
© 2009 by Pulmonary Hypertension Association. All rights reserved.<br />
<strong>No</strong>ne of the contents may be reproduced in any<br />
form whatsoever without the written permission of <strong>PHA</strong>.<br />
ISSN: 1933-088X (print); 1933-0898 (online)<br />
Editorial Offices<br />
Advances in Pulmonary Hypertension, DataMedica,<br />
P.O. Box 1688, Westhampton Beach, NY 11978<br />
Tel (631) 288-7733 Fax (631) 288-7744<br />
E-mail: sbelsonchapman@aol.com<br />
Publishing Staff<br />
Stu Chapman, Executive Editor<br />
Natalie Timoshin, Associate Editor<br />
Gloria Catalano, Production Director<br />
Michael McClain, Design Director<br />
Advances in Pulmonary Hypertension is circulated to cardiologists,<br />
pulmonologists, rheumatologists, and other selected<br />
physicians by the Pulmonary Hypertension Association. The<br />
contents are independently determined by the Editor and the<br />
Editorial Advisory Board. All past issues of the journal are available<br />
at: www.<strong>PHA</strong>ssociation.org/Medical/Advances_in_PH/<br />
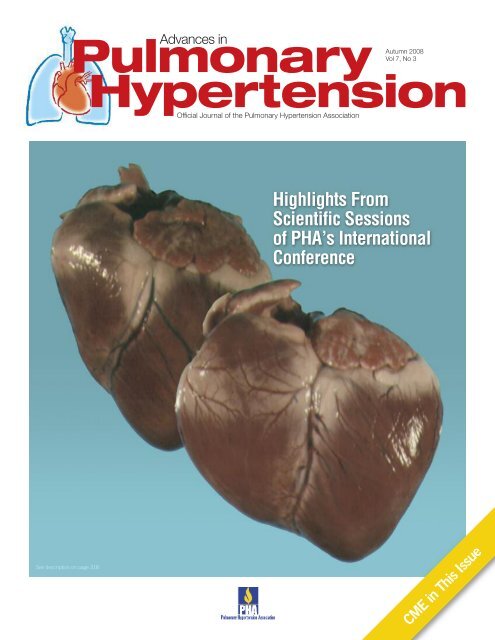
Cover Image<br />
Images suggest how severe pulmonary arterial hypertension<br />
can cause right ventricular dilatation and failure. (Images<br />
courtesy of Ivan McMurtry, PhD)<br />
Guest Editor’s Memo<br />
From <strong>PHA</strong>’s Scientific Sessions, a Time for<br />
Reflection on the Progress Toward a Cure<br />
As Guest Editor for this issue of Advances in Pulmonary Hypertension,<br />
I looked forward to reviewing the submission of manuscripts because<br />
I knew the content would reflect the exciting agenda we put together<br />
for the third Scientific Sessions held in conjunction with the 2008<br />
Pulmonary Hypertension Association (<strong>PHA</strong>) International Conference<br />
in Houston. As Chair of the Scientific Sessions Committee I had the<br />
privilege of overseeing the scope of the program and helping to coordinate<br />
content development. For readers who were fortunate enough to<br />
attend, the Scientific Sessions and conference once again offered an<br />
outstanding opportunity to meet with specialists in PH and explore why this program offers<br />
clinicians so much to think about and apply in their practices as they explore translational<br />
research in this disease.<br />
As researchers, we are always impressed and encouraged by the pace of work on this<br />
disease throughout the world and our content in this issue demonstrates some of the<br />
progress we are making in gaining a better understanding of the pathophysiology of PH,<br />
its mechanisms and treatment. Despite the progress in this regard, the attendance at the<br />
Conference by hundreds of patients and their families who signed up for the patient<br />
portion of he program reminded us of how much further we need to go before we can say<br />
we have a cure for PH. In achieving that goal, there will be numerous incremental steps<br />
such as the reports in this publication that serve as benchmarks for how far we have come<br />
on this huge journey.<br />
In this issue of the journal we express our gratitude to the following authors for their<br />
contributions to the growing body of knowledge on the disease: Heiko Bugger, MD, PhD<br />
and E. Dale Abel, MD, PhD, Paul M. Hassoun, MD, Kurt Stenmark MD, Hunter C. Champion,<br />
MD, PhD, and Ivan F. McMurtry, PhD. I woud also like to thank the participants in<br />
the Pulmonary Hypertension Roundtable Discusssion, including Todd Bull, MD, Omar<br />
Minai, MD, and Dr McMurtry.<br />
Karen A. Fagan, MD<br />
Guest Editor<br />
Editor’s Memo<br />
A few years after I began to work in the field of PH with my mentor,<br />
Dr. Bruce Brundage, I missed what would have been my 1 st International<br />
<strong>PHA</strong> conference in 1998: my son was born 2 days before the meeting<br />
started. At the time I did not know what I would be missing. At the<br />
following <strong>PHA</strong> conference, I found out what all the fuss was about.<br />
In 2000, over 700 patients, caregivers, practitioners and researchers<br />
converged on a sold-out hotel in suburban Chicago, and everyone poured<br />
their hearts (and minds) out to better the PH community. Children wearing<br />
backpacks with IV pumps inside, patients parading on stage showing<br />
off the latest pump-concealing fashions, and PH experts volunteering their time and<br />
expense to inform, teach and learn about PH were among the many highlights of that and<br />
each subsequent meeting I attended. To say that the biennial International Conferences<br />
and Scientific Sessions of the Pulmonary Hypertension Association are one of the most<br />
emotionally draining yet inspirational and uplifting events in the lives of anyone involved<br />
in PH would be a major understatement. This issue’s coverage of the most recent <strong>PHA</strong><br />
meeting, the 8 th International Conference and Scientific Sessions of the Pulmonary Hypertension<br />
Association thus holds a special place in my heart. Dr. Karen Fagan, Guest Editor<br />
of this issue and Chair of the <strong>PHA</strong> Scientific Sessions held in Houston last June, did a<br />
fantastic job putting together an entire issue devoted to the Conference, in which over<br />
1100 people from 17 different countries attended. From original scientific contributions<br />
to an expert Roundtable, all focused on the Scientific Sessions, plus an international<br />
commentary on PH and connective tissue disease issues covered in the last Summer issue<br />
of Advances, I am sure you too will learn and hopefully be inspired to attend the next<br />
International Conference.<br />
Ronald J. Oudiz, MD<br />
Editor-in-Chief
Editorial Advisory Board<br />
Editor-in-Chief<br />
Ronald J. Oudiz, MD<br />
Associate Professor of Medicine<br />
UCLA School of Medicine<br />
Director, Liu Center for Pulmonary<br />
Hypertension<br />
Division of Cardiology<br />
Los Angeles Biomedical Research<br />
Institute at Harbor-UCLA<br />
Medical Center<br />
Torrance, California<br />
Immediate Past Editor<br />
Vallerie V. McLaughlin, MD<br />
Associate Professor of Medicine<br />
Director, Pulmonary Hypertension<br />
Program<br />
<strong>University</strong> of Michigan Health System<br />
Ann Arbor, Michigan<br />
Editor-in-Chief Elect<br />
Richard Channick, MD<br />
Professor of Clinical Medicine<br />
Pulmonary and Critical Care Division<br />
<strong>University</strong> of California, San Diego Medical<br />
Center<br />
San Diego, California<br />
Associate Editors<br />
Erika Berman Rosenzweig, MD<br />
Assistant Professor of Pediatrics<br />
Department of Pediatrics<br />
Columbia College of Physicians<br />
and Surgeons<br />
New York, New York<br />
Todd Bull, MD<br />
Associate Professor of Medicine<br />
Medical Director, ICU Anshutz<br />
Inpatient Pavilion<br />
Division of Pulmonary Sciences and<br />
Critical Care Medicine<br />
<strong>University</strong> of Colorado Health Sciences<br />
Center<br />
Denver, Colorado<br />
Robert Schilz, DO, PhD<br />
Medical Director of Lung Transplantation<br />
and Pulmonary Vascular Disease<br />
<strong>University</strong> Hospital of Cleveland<br />
Case Western Reserve <strong>University</strong><br />
Cleveland, Ohio<br />
Editorial Board<br />
Teresa De Marco, MD<br />
Director, Heart Failure and<br />
Pulmonary Hypertension Program<br />
<strong>University</strong> of California, San Francisco<br />
San Francisco, California<br />
Eli Gabbay, MD<br />
Associate Professor<br />
<strong>University</strong> of Western Australia<br />
School of Medicine and Pharmacology<br />
Medical Director, Advanced Lung Disease<br />
and Pulmonary Vascular Unit<br />
Royal Perth Hospital<br />
Perth, Australia<br />
Kristin Highland, MD<br />
Assistant Professor<br />
Division of Pulmonary and Critical<br />
Care Medicine<br />
Director, Pulmonary Hypertension Clinic<br />
Medical <strong>University</strong> of South Carolina<br />
Charleston, South Carolina<br />
Omar Minai, MD<br />
Staff Physician<br />
Cleveland Clinic<br />
Cleveland, Ohio<br />
Myung H. Park, MD<br />
Director, Pulmonary Vascular<br />
Diseases Program<br />
<strong>University</strong> of Maryland School of<br />
Medicine<br />
Baltimore, Maryland<br />
Ioana Preston, MD<br />
Assistant Professor of Medicine<br />
Tufts-New England Medical Center<br />
Boston, Massachusetts<br />
Zeenat Safdar, MD<br />
Assistant Professor of Medicine<br />
Department of Medicine,<br />
Pulmonary & Critical Care Section<br />
Pulmonary Hypertension Center<br />
Baylor College of Medicine<br />
Houston, Texas<br />
Rajan Saggar, MD<br />
Assistant Professor of Medicine<br />
Division of Pulmonary and Critical Care<br />
Medicine and Hospitalists<br />
David Geffen School of<br />
Medicine at UCLA<br />
Los Angeles, California<br />
Francisco Soto, MD<br />
Assistant Professor<br />
Director, Pulmonary Hypertension<br />
Program<br />
Medical College of Wisconsin<br />
Milwaukee, Wisconsin<br />
Fernando Torres, MD<br />
Director, Pulmonary Hypertension<br />
Program<br />
UT Southwestern Medical Center<br />
Dallas, Texas<br />
Program Description<br />
The mission of Advances in Pulmonary Hypertension<br />
is to serve as the premiere<br />
forum for state of the art information regarding<br />
diagnosis, pathophysiology, and treatment of<br />
pulmonary hypertension. The 2003 Venice revision<br />
of the World Health Organization Classification<br />
serves as a guide to categories of<br />
pulmonary hypertension addressed by the Journal.<br />
While focusing on WHO Group I PAH, the<br />
other categories (Group II, Left heart<br />
disease; Group III, Associated with lung disease<br />
and/or hypoxemia; Group IV, Thrombotic<br />
and/or Embolic Disease; Group V, Miscellaneous)<br />
are also addressed. This mission is<br />
achieved by a combination of invited review articles,<br />
Roundtable discussions with panels<br />
consisting of international experts in PH, and<br />
original contributions. In addition, a special<br />
section entitled “Profiles in Pulmonary Hypertension”recognizes<br />
major contributors to the<br />
field and serves as an inspiring reminder of the<br />
rich and collegial history of dedication to advancing<br />
the field.<br />
Objectives<br />
• Provide up-to-date information regarding diagnosis,<br />
pathophysiology, and treatment<br />
of pulmonary hypertension.<br />
• Serve as a forum for presentation and discussion<br />
of important issues in the field, including<br />
new paradigms of disease<br />
understanding and investigational trial design.<br />
• Recognize and preserve the rich history of<br />
individuals who have made major contributions<br />
to the field via dedication to patient<br />
care, innovative research, and furthering the<br />
mission of the PH community to cure pulmonary<br />
hypertension.<br />
The Scientific Leadership<br />
Council of the Pulmonary<br />
Hypertension Association<br />
The scientific program of the Pulmonary<br />
Hypertension Association is guided by<br />
the association’s Scientific Leadership<br />
Council. The Council includes the<br />
following health care professionals:<br />
Vallerie V. McLaughlin, MD<br />
SLC Chair<br />
<strong>University</strong> of Michigan Health System<br />
Ann Arbor, Michigan<br />
David B. Badesch, MD<br />
SLC Immediate Past Chair<br />
<strong>University</strong> of Colorado Health<br />
Sciences Center<br />
Denver, Colorado<br />
John H. Newman, MD<br />
SLC Chair Elect<br />
Vanderbilt Medical School<br />
Nashville, Tennessee<br />
Robyn J. Barst, MD<br />
New York, New York<br />
Raymond L. Benza, MD<br />
<strong>University</strong> of Alabama Health System<br />
Birmingham, Alabama<br />
Todd Bull, MD<br />
<strong>University</strong> of Colorado Health<br />
Sciences Center<br />
Denver, Colorado<br />
Richard N. Channick, MD<br />
UCSD Medical Center<br />
San Diego, California<br />
C. Gregory Elliott, MD<br />
LDS Hospital<br />
<strong>University</strong> of Utah School of Medicine<br />
Salt Lake City, Utah<br />
Karen A. Fagan, MD<br />
<strong>University</strong> of South Alabama<br />
College of Medicine<br />
Mobile, Alabama<br />
Adaani Frost, MD<br />
Baylor College of Medicine<br />
Houston, Texas<br />
John Granton, MD<br />
Toronto General Hospital<br />
Toronto, Canada<br />
Nazzareno Galiè, MD<br />
Institute of Cardiology<br />
<strong>University</strong> of Bologna<br />
Bologna, Italy<br />
Nicholas S. Hill, MD<br />
Division of Pulmonary, Critical Care<br />
and Sleep Medicine<br />
Tufts-New England Medical Center<br />
Boston, Massachusetts<br />
Marius Hoeper, MD<br />
Hannover Medical school<br />
Hannover, Germany<br />
Dunbar Ivy, MD<br />
<strong>University</strong> of Colorado Health<br />
Sciences Center<br />
Denver, Colorado<br />
Zhi-Cheng Jing, MD<br />
Fu wai Heart Hospital<br />
Beijing, China<br />
Anne M. Keogh, MD<br />
St. Vincent’s Public Hospital<br />
Sydney, Australia<br />
Michael J. Krowka, MD<br />
Mayo Clinic<br />
Rochester, Minnesota<br />
James E. Loyd, MD<br />
Vanderbilt <strong>University</strong> Medical Center<br />
Nashville, Tennessee<br />
Michael D. McGoon, MD<br />
Chair, <strong>PHA</strong> Board of Trustees<br />
Pulmonary Hypertension Clinic<br />
Mayo Clinic<br />
Rochester, Minnesota<br />
Srinivas Murali, MD<br />
Allegheny General Hospital<br />
Pittsburgh, Pennsylvania<br />
Ronald J. Oudiz, MD<br />
Liu Center for Pulmonary Hypertension<br />
Los Angeles Biomedical Research<br />
Institute<br />
Harbor-UCLA Medical Center<br />
Torrance, California<br />
Marlene Rabinovitch, MD<br />
Stanford <strong>University</strong> School of Medicine<br />
Stanford, California<br />
Erica Berman-Rosenzweig, MD<br />
Columbia-Presbyterian Medical Center<br />
New York, New York<br />
Ivan M. Robbins, MD<br />
SLC Scientific Sessions Committee<br />
Vanderbilt <strong>University</strong><br />
Nashville, Tennessee<br />
Julio Sandoval, MD<br />
Cardiopulmonary Department<br />
National Institute of Cardiology<br />
of Mexico<br />
Tlalpan, Mexico<br />
Richard Silver, MD<br />
Medical <strong>University</strong> of South Carolina<br />
Charleston, South Carolina<br />
Victor F. Tapson, MD<br />
Division of Pulmonary and Critical<br />
Care Medicine<br />
Duke <strong>University</strong> Medical Center<br />
Durham, <strong>No</strong>rth Carolina<br />
Liaisons<br />
Arlene Schiro, RN, MA, ACNP-BC<br />
Chair, PH Resource Network<br />
Massachusetts General Hospital<br />
Boston, Massachusetts<br />
Joanne Sperando Schmidt<br />
Patient Liaison<br />
Emeritus Members<br />
Bruce H. Brundage, MD<br />
St. Charles Medical Center-Bend<br />
Bend, Oregon<br />
Alfred P. Fishman, MD<br />
<strong>University</strong> of Pennsylvania Health<br />
System<br />
Philadelphia, Pennsylvania<br />
The Mission of the Scientific Leadership<br />
Council is to provide medical and scientific<br />
guidance and support to the <strong>PHA</strong> by:<br />
• Developing and disseminating knowledge<br />
for diagnosing and treating pulmonary<br />
hypertension<br />
• Advocating for patients with pulmonary hypertension<br />
• Increasing involvement of basic and clinical<br />
researchers and practitioners<br />
More information on <strong>PHA</strong>’s Scientific<br />
Leadership Council and associated<br />
committees can be found at:<br />
www.<strong>PHA</strong>ssociation.org/SLC/<br />
Advances in Pulmonary Hypertension 319
In a Remarkably Short Time,<br />
Vallerie McLaughlin, MD, Joins<br />
a Select Group of Investigators<br />
Blazing a Trail in PH Research<br />
Vallerie V.<br />
McLaughlin, MD<br />
The career path of Vallerie V. McLaughlin,<br />
MD, appeared clearly headed toward<br />
her choice of echocardiography until opportunity<br />
knocked on her office door in<br />
the form of Stuart Rich, MD, one of the<br />
country’s foremost experts on pulmonary<br />
hypertension (PH). Dr Rich offered her<br />
the chance to work with him on the PH<br />
service at the <strong>University</strong> of Illinois Hospital<br />
and Clinics in Chicago. Fresh from<br />
a cardiology fellowship at <strong>No</strong>rthwestern<br />
<strong>University</strong> and from advanced training and research in<br />
echocardiography, Dr McLaughlin recognized the compelling<br />
opportunity to work with PH patients at a critical<br />
juncture in their care, soon after the emergence of prostacyclin<br />
treatment.<br />
Accepting the offer, Dr McLaughlin embarked on a remarkable<br />
journey, first under the tutelage of Dr Rich and<br />
in the last 10 years during which she has been an integral<br />
part of every pivotal trial in PH as the spectrum of therapy<br />
grew significantly. Those first PH patients, however, provided<br />
the initial momentum for her commitment to the<br />
field. “They came to us so short of breath and we were<br />
able to help them with epoprostenol. It was a rewarding<br />
opportunity to make them better.” As an Associate Professor<br />
of Medicine at Rush Medical College, Chicago,<br />
Dr McLaughlin then became Associate Director, Rush<br />
Heart Institute Center of Pulmonary Heart Disease.<br />
“Everyone comes to a time when they need to take<br />
off on their own and it was time for me to venture out,”<br />
she recalled, citing her next opportunity to become director<br />
of the pulmonary hypertension program at the <strong>University</strong><br />
of Michigan. The program at Michigan has become<br />
one of the leading PH centers in the country. The author<br />
or co-author of more than 60 peer-reviewed articles,<br />
Dr McLaughlin is now the Principal Investigator of the<br />
Data Coordinating Center and Chairperson of the Steering<br />
Committee for the Pulmonary Hypertension Breakthrough<br />
Initiative, supported by The Cardiovascular Medical Research<br />
and Education Fund (CMREF). The CMREF mission<br />
is the support of research to uncover the etiology and<br />
pathogenesis of idiopathic pulmonary arterial hypertension<br />
(IPAH, or PPH), in pursuit of the ultimate goal of its treatment<br />
and cure.<br />
The Initiative procures the lungs of patients with PH<br />
who are undergoing a lung transplant, processes them,<br />
and distributes them for scientific study, according to<br />
Dr McLaughlin. “We hope that by getting diseased lungs<br />
into researchers’ hands we can make a real breakthrough<br />
in this disease. The main research interest of this project<br />
is basic science—including the pathology, proteomics,<br />
and genomics of PH. We have a center that is culturing<br />
endothelial, smooth muscle, and adventitial cells so we<br />
can do in vitro work with actual cells from PH patients.”<br />
Continuing her commitment to the programs of the<br />
Pulmonary Hypertension Association, Dr McLaughlin last<br />
year became the chair of <strong>PHA</strong>’s Scientific Leadership<br />
Council. Helping to coordinate <strong>PHA</strong>’s educational initiatives<br />
and multi-industry support, she oversees a broad<br />
range of initiatives such as the 30-city tour of educational<br />
programs, a preceptorship program, an online component<br />
offering different educational tracks for specialists and<br />
generalists and various regional events for patients and<br />
physicians.<br />
Despite her leadership position as Principal Investigator<br />
on numerous trials and her role in spearheading other<br />
research and educational activities, Dr McLaughlin is<br />
quick to offer her appreciation to colleagues who she says<br />
have served her so well as mentors and role models. Comments<br />
from some of these colleagues are among the<br />
following tributes offered to Dr McLaughlin.<br />
“Val is a highly dedicated PH physician and investigator.<br />
She is clearly loved by her patients, and she’s highly respected<br />
by her colleagues in the field. I don’t know quite<br />
how she does it all – she travels extensively, and yet is<br />
somehow able to serve as the director of a highly successful<br />
PH program at the <strong>University</strong> of Michigan, lead an<br />
effort to develop a comprehensive consensus statement for<br />
the American College of Cardiology, help to coordinate the<br />
PAH Breakthrough Initiative, serve as Chair of the Scientific<br />
Leadership Council for the <strong>PHA</strong>, and be a mom. There<br />
simply are not enough hours in the day to do this, and I<br />
happen to know that Val works through much of the night<br />
and on weekends as well – having received emails sent at<br />
some very early morning hours, and throughout the weekend.<br />
We are all very fortunate to have such a highly<br />
talented, hardworking, and dedicated colleague.”<br />
—David Badesch, MD<br />
“Val McLaughlin’s dedication to advancing research in<br />
pulmonary hypertension, educating others in the field, and<br />
her incredible energy and drive have been an inspiration<br />
to many, including me. The numerous, widely quoted<br />
papers bearing her name as first author testify to her importance<br />
in the field of clinical research in pulmonary<br />
hypertension. Although she is chronologically my junior,<br />
she has, in fact been a valued mentor!”<br />
—Richard Channick, MD<br />
“I first got to know Vallerie during the 2nd WHO PPH Sym-<br />
320 Advances in Pulmonary Hypertension
Advances in Pulmonary Hypertension<br />
Author Guidelines 2008<br />
Scope of Manuscripts<br />
Advances in Pulmonary Hypertension considers<br />
the following types of manuscripts for publication:<br />
• Reviews that summarize and synthesize peerreviewed<br />
literature to date on relevant topics in a<br />
scholarly fashion and format.<br />
• Letters to the Editor<br />
• Clinical Case Studies<br />
Manuscript Submission<br />
Authors are required to submit their manuscripts<br />
in an electronic format, preferably by email to<br />
the Editor-in-Chief, Richard Channick, MD,<br />
rchannick@ucsd.edu. Please provide manuscripts<br />
in a word processing program. Images should be<br />
submitted electronically as well.<br />
All material reproduced from previously published,<br />
copyrighted material should contain a full credit line<br />
acknowledging the original source. Authors are responsible<br />
for obtaining permission to reproduce<br />
such material.<br />
Contact Information: List all authors, including mailing<br />
address, titles and affiliations, phone, fax, and email.<br />
Please note corresponding author.<br />
Peer Review and Editing: Manuscripts will be peer reviewed.<br />
Accepted manuscripts will be edited for clarity,<br />
spelling, punctuation, grammar, and consistency with<br />
American Medical Association (AMA) style.<br />
Manuscript Preparation<br />
Length: Full-length manuscripts should not exceed<br />
4,000 words, including references. Please limit the<br />
reference list to 50 citations. Manuscripts should be<br />
accompanied by figures and/or tables. Generally, 4 to 5<br />
figures and 2 to 3 tables are preferred for each manuscript.<br />
Please include a brief description to accompany<br />
these items, as well as a key for all abbreviated<br />
words.<br />
Spacing: One space after commas and periods. Manuscripts<br />
should be double spaced. Manuscripts should<br />
not contain an abstract but an introduction is recommended.<br />
References: All submissions should include numbered<br />
references that are referred to in the text by superscripts<br />
and that conform to AMA style. Example:<br />
Lewczuk J, Piszko P, Jagas J, et al. Prognostic factors<br />
in medically treated patients with chronic pulmonary<br />
embolism. Chest. 2001;119:818-823.<br />
Copyright: Manuscripts and accompanying material<br />
are accepted for exclusive publication in Advances in<br />
Pulmonary Hypertension. <strong>No</strong>ne of the contents may<br />
be reproduced without permission of the Pulmonary<br />
Hypertension Association. To request permission,<br />
please contact Donica Merhazion, <strong>PHA</strong> Associate<br />
Director of Medical Services, 240 485 0744 or<br />
Donica@phassociation.org<br />
posium in Evian in 1998. It took no time whatsoever to<br />
know she had what it takes to become a top leader in the<br />
PH field. In addition to being bright, she was, and still is,<br />
incredibly compassionate, a critical thinker who does not<br />
take the written word as carte blanche but at the same<br />
time is truly modest regarding her significant contributions<br />
to the field. She knows how to ask questions, and question<br />
what may be considered “accepted” with grace. I have had<br />
the great pleasure to watch her grow and flourish. And although<br />
she has an enormous amount on her plate at one<br />
time, she never neglects any commitment she makes; and<br />
yet, she maintains the equipoise to know how important<br />
her children and family are to her, and she to them. I am<br />
not saying that what Vallerie has been able to accomplish<br />
is easy but I am certain that she has done it not for the accolades<br />
from others but because she is truly passionate<br />
about her career as she is about her family; and with her<br />
passion, she is able to make it work!!”—Robyn Barst, MD<br />
“Dr McLaughlin is a true leader in the field of pulmonary<br />
hypertension. She is involved in many of the important<br />
projects ongoing in this area and her commitment to the<br />
<strong>PHA</strong> is unwavering. I have had the opportunity to work<br />
with Val during my tenure on the SLC of the <strong>PHA</strong> and have<br />
always been impressed by her ability to quickly identify the<br />
key elements of any perceived problem or plan and at her<br />
skill at providing potential solutions and improvements.<br />
Her prominence in the field is evidenced by her authorship<br />
on many of the important papers and position statements<br />
that have been recently published and her skill as a physician<br />
is highlighted by the respect of her peers and the testimony<br />
of her patients. Personally, she has been an<br />
important mentor in my career and I am very happy to see<br />
her receive this well deserved recognition. The interesting<br />
thing is she is just now hitting her stride so we will continue<br />
to see great contributions from Val for years to<br />
come.”—Todd Bull, MD ■<br />
Advances in Pulmonary Hypertension 321
Building Medical<br />
Education in PH:<br />
A Partnership Initiative to<br />
Advance Medical Understanding of<br />
Pulmonary Hypertension<br />
Building Medical Education in PH events are designed<br />
to foster partnerships between <strong>PHA</strong> and PH Centers to<br />
promote continuing education in the field of Pulmonary<br />
Hypertension through CME educational events.<br />
Upcoming Events for<br />
Medical Professionals Include:<br />
February 13, 2009<br />
Warrensville Heights, OH<br />
PAH in the Real World: Managing<br />
the Aspects of a Frequently<br />
Missed Diagnosis Symposium<br />
<strong>University</strong> Hospitals Case<br />
Medical Center<br />
Pulmonary Arterial Hypertension is a frequently misunderstood<br />
diagnosis. This one-day symposium will offer lectures, case studies<br />
and open discussions with key opinion leaders in the area of<br />
pulmonary hypertension. After attending this symposium, one<br />
should better understand this unusual disease process. Visit<br />
http://cme.case.edu or call 216-983-1239.<br />
March 13 – 14, 2009<br />
San Francisco, CA<br />
2nd International Conference<br />
on Neonatal and Childhood<br />
Pulmonary Vascular Disease<br />
<strong>University</strong> of California,<br />
San Francisco<br />
It is increasingly clear that pulmonary vascular pathology is integral<br />
to a number of childhood disorders. In this symposium, we will<br />
bring together international experts to explore our current understanding<br />
of the basic pathobiology as well as new and future<br />
therapies for neonatal, pediatric and adult pulmonary vascular<br />
disease. Visit https://www.cme.ucsf.edu/cme/ or call 415-476-4251.<br />
June 4, 2009<br />
Hartford, CT<br />
3rd Annual Pulmonary<br />
Hypertension Symposium<br />
Yale <strong>University</strong> School of Medicine<br />
This one-day symposium will provide current educational information<br />
to properly diagnosis and treat pulmonary hypertension.<br />
More information will be available in 2009. To view the agenda<br />
from Yale’s 2nd Annual PH Symposium visit<br />
http://cme.yale.edu/conferences/.<br />
To partner with <strong>PHA</strong> in Building Medical Education in PH for<br />
your upcoming CME event, please contact Jennie Carman,<br />
Meetings Planning Associate, at 301-565-3004 x763 or<br />
BME@<strong>PHA</strong>ssociation.org.
SECOND<br />
WIND<br />
IN PAH
FLOLAN:<br />
Over a Decade of<br />
Experience in PAH<br />
INDICATION: FLOLAN is indicated for the long-term intravenous treatment of primary pulmonary hypertension and pulmonary hypertension associated<br />
with the scleroderma spectrum of disease in NYHA Class III and Class IV patients who do not respond adequately to conventional therapy.<br />
IMPORTANT SAFETY INFORMATION: Chronic use of FLOLAN is contraindicated in patients with congestive heart failure due to severe left ventricular<br />
systolic dysfunction.<br />
FLOLAN should not be used chronically in patients who develop pulmonary edema during dose initiation.<br />
FLOLAN must be reconstituted only as directed using STERILE DILUENT for FLOLAN. FLOLAN must not be reconstituted or mixed with any other<br />
parenteral medications or solutions prior to or during administration.<br />
Abrupt withdrawal or reductions in delivery of FLOLAN, as well as overdoses, may result in hemodynamic instability, including rebound pulmonary<br />
hypertension or fatal hypotension.<br />
FLOLAN should be used only by clinicians experienced in the diagnosis and treatment of pulmonary hypertension.<br />
FLOLAN is a potent inhibitor of platelet aggregation. Therefore, an increased risk for hemorrhagic complications should be considered, particularly for<br />
patients with other risk factors for bleeding.<br />
During chronic use, FLOLAN is delivered continuously on an ambulatory basis through a permanent indwelling central venous catheter. Unless<br />
contraindicated, anticoagulant therapy should be administered to PPH and PH/SSD patients receiving FLOLAN to reduce the risk of pulmonary<br />
thromboembolism or systemic embolism through a patent foramen ovale. In order to reduce the risk of infection, aseptic technique must be used in the<br />
reconstitution and administration of FLOLAN as well as in routine catheter care. Dosage of FLOLAN during chronic use should be adjusted at the first<br />
sign of recurrence or worsening of symptoms.<br />
Chronic adverse events reported during clinical trials include headache, jaw pain, flushing, diarrhea, nausea and vomiting, flu-like symptoms,<br />
and anxiety/nervousness.<br />
Serious adverse events have been reported during post-approval use of FLOLAN. These include sepsis, anemia, hypersplenism, thrombocytopenia,<br />
pancytopenia, splenomegaly, and hyperthyroidism.<br />
Excessive doses of FLOLAN may acutely result in systemic hypotension, tachycardia, headache, flushing, nausea and vomiting, or diarrhea; excessive<br />
doses administered chronically can lead to the development of a hyperdynamic state and high-output cardiac failure.*<br />
* Badesch DB, Abman SH, Ahearn GS, et al. Medical therapy for pulmonary arterial hypertension:<br />
ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1, suppl):35S-62S.<br />
Please see adjacent page for brief summary of full prescribing information.<br />
© 2008 Gilead Sciences, Inc. All rights reserved. FLO07203PAD May 2008<br />
Gilead and the Gilead logo are trademarks of Gilead Sciences, Inc.<br />
FLOLAN is a registered trademark of GlaxoSmithKline Group of Companies.
FLOLAN ® (epoprostenol sodium) for Injection<br />
Brief Summary of full prescribing information. See full prescribing information. Rx only.<br />
INDICATIONS AND USAGE:<br />
FLOLAN is indicated for the long-term intravenous treatment of primary pulmonary hypertension and pulmonary<br />
hyperten sion associated with the scleroderma spectrum of disease in NYHA Class III and Class IV patients who do<br />
not respond ade quately to conventional therapy.<br />
CONTRAINDICATIONS:<br />
The chron ic use of FLOLAN in patients with congestive heart failure due to severe left ventricular systolic dysfunction<br />
is therefore con traindicated. Some patients with pulmonary hypertension have developed pulmonary edema during<br />
dose initiation, which may be asso ciated with pulmonary veno-occlusive disease. FLOLAN should not be used<br />
chronically in patients who develop pulmonary edema during dose initiation. FLOLAN is also contraindicated in patients<br />
with known hypersensitivity to the drug or to structurally related compounds.<br />
WARNINGS:<br />
FLOLAN must be reconstituted only as directed using Sterile Diluent for FLOLAN. FLOLAN must not be reconstituted<br />
or mixed with any other parenteral medications or solutions prior to or during administration. Abrupt<br />
Withdrawal: Abrupt withdrawal (including interruptions in drug delivery) or sudden large reductions in dosage of FLOLAN<br />
may result in symptoms associated with rebound pulmonary hypertension, including dyspnea, dizziness, and asthenia. In<br />
clinical trials, one Class III PPH patient’s death was judged attributable to the interruption of FLOLAN. Abrupt withdrawal<br />
should be avoided. Sepsis: See ADVERSE REACTIONS: Adverse Events Attributable to the Drug Delivery System.<br />
PRECAUTIONS:<br />
General: FLOLAN should be used only by clinicians experienced in the diagnosis and treatment of pulmonary hypertension.<br />
FLOLAN is a potent pulmonary and systemic vasodilator. Dose initiation with FLOLAN must be performed in a<br />
setting with adequate personnel and equipment for physiologic monitoring and emergency care. Dose initiation in<br />
controlled PPH clini cal trials was performed during right heart catheterization. In uncontrolled PPH and controlled<br />
PH/SSD clinical trials, dose initiation was performed without cardiac catheterization. The risk of cardiac catheterization<br />
in patients with pulmonary hyper tension should be carefully weighed against the potential benefi ts. During dose initiation,<br />
asymptomatic increases in pul monary artery pressure coincident with increases in cardiac output occurred rarely.<br />
In such cases, dose reduction should be considered, but such an increase does not imply that chronic treatment is<br />
contraindicated. FLOLAN is a potent inhibitor of platelet aggregation. Therefore, an increased risk for hemorrhagic<br />
complications should be considered, particularly for patients with other risk factors for bleeding (see PRECAUTIONS:<br />
Drug Interactions). During chronic use, FLOLAN is delivered continuously on an ambulatory basis through a permanent<br />
indwelling central venous catheter. Unless contraindicated, anticoagulant therapy should be administered to PPH and<br />
PH/SSD patients receiv ing FLOLAN to reduce the risk of pulmonary thromboembolism or systemic embolism through a<br />
patent foramen ovale. In order to reduce the risk of infection, aseptic technique must be used in the reconstitution and<br />
administration of FLOLAN as well as in routine catheter care. Because FLOLAN is metabolized rapidly, even brief interruptions<br />
in the delivery of FLOLAN may result in symptoms associated with rebound pulmonary hypertension including<br />
dyspnea, dizziness, and asthenia. The decision to initiate therapy with FLOLAN should be based upon the understanding<br />
that there is a high likelihood that intra venous therapy with FLOLAN will be needed for prolonged periods, possibly<br />
years, and the patient’s ability to accept and care for a permanent intravenous catheter and infusion pump should be<br />
carefully considered. Dosage of FLOLAN during chronic use should be adjusted at the fi rst sign of recurrence or worsening<br />
of symptoms attributable to pulmonary hypertension or the occurrence of adverse events associated with FLOLAN<br />
(see DOSAGE AND ADMINISTRATION). Following dosage adjustments, standing and supine blood pressure and heart<br />
rate should be monitored closely for several hours. Information for Patients: Patients receiving FLOLAN should receive<br />
the following information. FLOLAN must be recon stituted only with Sterile Diluent for FLOLAN. FLOLAN is infused<br />
continuously through a permanent indwelling central venous catheter via a small, portable infusion pump. Thus, therapy<br />
with FLOLAN requires commitment by the patient to drug reconstitution, drug administration, and care of the permanent<br />
central venous catheter. Sterile technique must be adhered to in preparing the drug and in the care of the<br />
catheter, and even brief interruptions in the delivery of FLOLAN may result in rapid symptomatic deterioration. A patient’s<br />
decision to receive FLOLAN should be based upon the understanding that there is a high likelihood that therapy<br />
with FLOLAN will be needed for prolonged periods, possibly years. The patient’s ability to accept and care for a permanent<br />
intravenous catheter and infusion pump should also be carefully considered. Drug Interactions: Additional reductions<br />
in blood pressure may occur when FLOLAN is administered with diuretics, antihypertensive agents, or other vasodilators.<br />
When other antiplatelet agents or anticoagulants are used concomitantly, there is the potential for FLOLAN to<br />
increase the risk of bleeding. However, patients receiving infusions of FLOLAN in clinical trials were maintained on<br />
anticoagulants without evidence of increased bleeding. In clinical trials, FLOLAN was used with digoxin, diuretics, anticoagulants,<br />
oral vasodilators, and supplemental oxygen. In a pharmacokinetic substudy in patients with congestive<br />
heart failure receiving furosemide or digoxin in whom therapy with FLOLAN was initiated, apparent oral clearance values<br />
for furosemide (n = 23) and digoxin (n = 30) were decreased by 13% and 15%, respectively, on the second day of<br />
therapy and had returned to baseline values by day 87. The change in furosemide clearance value is not likely to be<br />
clinically signifi cant. However, patients on digoxin may show elevations of digoxin concentrations after initiation of<br />
therapy with FLOLAN, which may be clinically signifi cant in patients prone to digoxin toxicity. Carcinogenesis, Mutagenesis,<br />
Impairment of Fertility: Long-term studies in animals have not been performed to evaluate carcinogenic<br />
potential. A micronucleus test in rats revealed no evidence of mutagenicity. The Ames test and DNA elution tests were<br />
also negative, although the instability of epoprostenol makes the signifi cance of these tests uncertain. Fertility was not<br />
impaired in rats given FLOLAN by subcutaneous injection at doses up to 100 mcg/kg/day (600 mcg/m 2 /day, 2.5 times<br />
the recommended human dose [4.6 ng/kg/min or 245.1 mcg/m 2 /day, IV] based on body surface area). Pregnancy:<br />
Pregnancy Category B. Reproductive studies have been performed in pregnant rats and rabbits at doses up to<br />
100 mcg/kg/day (600 mcg/m 2 /day in rats, 2.5 times the recommended human dose, and 1,180 mcg/m 2 /day in rabbits,<br />
4.8 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility<br />
or harm to the fetus due to FLOLAN. There are, however, no adequate and well-controlled studies in pregnant women.<br />
Because animal reproduction studies are not always predictive of human response, this drug should be used during<br />
pregnancy only if clearly needed. Labor and Delivery: The use of FLOLAN during labor, vaginal delivery, or cesarean<br />
section has not been adequately studied in humans. Nursing Mothers: It is not known whether this drug is excreted in<br />
human milk. Because many drugs are excreted in human milk, caution should be exercised when FLOLAN is administered<br />
to a nursing woman. Pediatric Use: Safety and effectiveness in pediatric patients have not been established.<br />
Geriatric Use: Clinical studies of FLOLAN in pulmonary hypertension did not include suffi cient numbers of subjects<br />
aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience<br />
has not identifi ed differences in responses between the elderly and younger patients. In general, dose selection<br />
for an elderly patient should be cautious, usually starting at the low end of the dosing range, refl ecting the greater<br />
frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.<br />
ADVERSE REACTIONS:<br />
During clinical trials, adverse events were classifi ed as follows: (1) adverse events during dose initiation and escalation,<br />
(2) adverse events during chronic dosing, and (3) adverse events associated with the drug delivery system.<br />
Adverse Events During Dose Initiation and Escalation: During early clinical trials, FLOLAN was increased in 2-ng/<br />
kg/min increments until the patients developed symptomatic intolerance. The most common adverse events and the<br />
adverse events that limited further increases in dose were generally related to vasodilation, the major pharmacologic<br />
effect of FLOLAN. The most common dose-limiting adverse events (occurring in ≥1% of patients) were nausea, vomiting,<br />
headache, hypoten sion, and fl ushing, but also include chest pain, anxiety, dizziness, bradycardia, dyspnea, abdominal<br />
pain, musculoskeletal pain, and tachycardia. Adverse events reported in ≥1% of patients receiving FLOLAN<br />
(n = 391) during dose initiation and escalation are: fl ushing 58%; headache 49%; nausea/vomiting 32%; hypotension<br />
16%; anxiety, nervousness, agitation 11%; chest pain 11%; dizziness 8%; bradycardia 5%; abdominal pain 5%; musculoskeletal<br />
pain 3%; dyspnea 2%; back pain 2%; sweating 1%; dyspepsia 1%; hypesthesia/paresthesia 1%; and<br />
t ach y c ar dia 1%. Adverse Events During Chronic Administration: Interpretation of adverse events is complicated by<br />
the clinical features of PPH and PH/SSD, which are similar to some of the pharmacologic effects of FLOLAN (e.g., dizziness,<br />
syncope). Adverse events probably related to the underlying disease include dyspnea, fatigue, chest pain, edema,<br />
hypoxia, right ventricular fail ure, and pallor. Several adverse events, on the other hand, can clearly be attributed to<br />
FLOLAN. These include headache, jaw pain, fl ushing, diarrhea, nausea and vomiting, fl u-like symptoms, and anxiety/<br />
nervousness. Adverse Events During Chronic Administration for PPH: In an effort to separate the adverse effects of<br />
the drug from the adverse effects of the underlying disease, the following is a listing of adverse events that occurred at<br />
a rate at least 10% dif ferent in the 2 groups [FLOLAN (n = 52), conventional therapy (n = 54)] in controlled trials for<br />
PPH (events are listed by incidence for FLOLAN followed by conventional therapy): Occurrence More Common with<br />
FLOLAN: General: chills/fever/sepsis/fl u-like symptoms (25%, 11%); Cardiovascular: tachycardia (35%, 24%), fl ushing<br />
(42%, 2%); Gastrointestinal: diarrhea (37%, 6%), nausea/vomiting (67%, 48%); Musculoskeletal: jaw pain<br />
(54%, 0%), myalgia (44%, 31%), nonspecifi c musculoskeletal pain (35%, 15%); Neurological: anxiety/nervousness/<br />
tremor (21%, 9%), dizziness (83%, 70%), headache (83%, 33%), hypesthesia, hyper esthesia, paresthesia (12%, 2%).<br />
Occurrence More Common With Standard Therapy: Cardiovascular: heart failure (31%, 52%), syncope (13%,<br />
24%), shock (0%, 13%); Respiratory: hypoxia (25%, 37%). Thrombocytopenia has been reported during uncontrolled<br />
clinical trials in patients receiving FLOLAN.<br />
Additional adverse events that occurred at a rate with less than 10% difference reported in PPH patients receiving<br />
FLOLAN ® (epoprostenol sodium) for injection plus conventional therapy (n = 52) compared to conventional therapy alone<br />
(n = 54) during controlled clinical trials (events are listed by incidence for FLOLAN followed by conventional therapy):<br />
General: asthenia (87%, 81%); Cardiovascular: angina pectoris (19%, 20%), arrhythmia (27%, 20%), bradycardia<br />
(15%, 9%), supraventricu lar tachycardia (8%, 0%), pallor (21%, 30%), cyanosis (31%, 39%), palpitation (63%, 61%),<br />
cerebrovascular accident (4%, 0%), hemorrhage (19%, 11%), hypotension (27%, 31%), myocardial ischemia (2%,<br />
6%); Gastrointestinal: abdominal pain (27%, 31%), anorexia (25%, 30%), ascites (12%, 17%), constipation (6%,<br />
2%); Metabolic: edema (60%, 63%), hypokalemia (6%, 4%), weight reduction (27%, 24%), weight gain (6%, 4%);<br />
Musculoskeletal: arthralgia (6%, 0%), bone pain (0%, 4%), chest pain (67%, 65%); Neurological: confusion (6%,<br />
11%), convulsion (4%, 0%), depression (37%, 44%), insomnia (4%, 4%); Respiratory: cough increase (38%, 46%),<br />
dyspnea (90%, 85%), epistaxis (4%, 2%), pleural effusion (4%, 2%); Skin and Appendages: pruritus (4%, 0%), rash<br />
(10%, 13%), sweating (15%, 20%); Special Senses: amblyopia (8%, 4%), vision abnormality (4%, 0%). Adverse<br />
Events During Chronic Administration for PH/SSD: In an effort to separate the adverse effects of the drug from the<br />
adverse effects of the underlying disease, the following is a listing of adverse events that occurred at a rate at least<br />
10% different in the 2 groups [FLOLAN (n = 56) and conventional therapy (n = 55)] in the controlled trial for patients<br />
with PH/SSD (events are listed by incidence for FLOLAN followed by conventional therapy): Occurrence More Common<br />
With FLOLAN: Cardiovascular: fl ushing (23%, 0%), hypotension (13%, 0%); Gastrointestinal: anorexia (66%, 47%),<br />
nausea/vomiting (41%, 16%), diarrhea (50%, 5%); Musculoskeletal: jaw pain (75%, 0%), pain/neck pain/arthralgia<br />
(84%, 65%); Neurological: headache (46%, 5%); Skin and Appendages: skin ulcer (39%, 24%), eczema/rash/urticaria<br />
(25%, 4%). Occurrence More Common With Conventional Therapy: Cardiovascular: cyanosis (54%, 80%),<br />
pallor (32%, 53%), syncope (7%, 20%); Gastrointestinal: ascites (23%, 33%), esophageal refl ux/gastritis (61%,<br />
73%); Metabolic: weight decrease (45%, 56%); Neurological: dizziness (59%, 76%); Respiratory: hypoxia (55%,<br />
65%). Additional adverse events that occurred at a rate with less than 10% difference reported in PH/SSD patients<br />
receiving FLOLAN plus conventional therapy (n = 56) or conventional therapy alone (n = 55) during controlled clinical<br />
trials (adverse events occurred in at least 2 patients in either treatment group and are listed by inci dence for FLOLAN<br />
followed by conventional therapy): General: asthenia (100%, 98%), hemorrhage/hemorrhage injection site/hemorrhage<br />
rectal (11%, 2%), infection/rhinitis (21%, 20%), chills/fever/sepsis/fl u-like symptoms (13%, 11%); Blood and<br />
Lymphatic: thrombocytopenia (4%, 0%); Cardiovascular: heart failure/heart failure right (11%, 13%), myocardial<br />
infarction (4%, 0%), palpitation (63%, 71%), shock (5%, 5%), tachycardia (43%, 42%), vascular disorder peripheral<br />
(96%, 100%), vascular disorder (95%, 89%); Gastrointestinal: abdominal enlargement (4%, 0%), abdominal pain<br />
(14%, 7%), con stipation (4%, 2%), fl atulence (5%, 4%); Metabolic: edema/edema peripheral/edema genital (79%,<br />
87%), hypercalcemia (48%, 51%), hyperkalemia (4%, 0%), thirst (0%, 4%); Musculoskeletal: arthritis (52%, 45%),<br />
back pain (13%, 5%), chest pain (52%, 45%), cramps leg (5%, 7%); Respiratory: cough increase (82%, 82%), dyspnea<br />
(100%, 100%), epistaxis (9%, 7%), pharyngitis (5%, 2%), pleural effusion (7%, 0%), pneumonia (5%, 0%),<br />
pneumothorax (4%, 0%), pulmonary edema (4%, 2%), respiratory disorder (7%, 4%), sinusitis (4%, 4%); Neurological:<br />
anxiety/hyperkinesia/nervousness/tremor (7%, 5%), depression/depression psychotic (13%, 4%), hyperesthesia/<br />
hypesthesia/paresthesia (5%, 0%), insomnia (9%, 0%), somnolence (4%, 2%); Skin and Appendages: collagen disease<br />
(82%, 84%), pruritus (4%, 2%), sweat (41%, 36%); Urogenital: hematuria (5%, 0%), urinary tract infection (7%,<br />
0%). Although the relationship to FLOLAN administration has not been established, pulmonary embolism has been reported<br />
in several patients taking FLOLAN and there have been reports of hepatic failure. Adverse Events Attributable<br />
to the Drug Delivery System: Chronic infusions of FLOLAN are delivered using a small, portable infusion pump through<br />
an indwelling central venous catheter. During controlled PPH trials of up to 12 weeks’ dura tion, up to 21% of patients<br />
reported a local infection and up to 13% of patients reported pain at the injection site. During a controlled PH/SSD<br />
trial of 12 weeks’ duration, 14% of patients reported a local infection and 9% of patients reported pain at the injection<br />
site. During long-term follow-up in the clinical trial of PPH, sepsis was reported at least once in 14% of patients and<br />
occurred at a rate of 0.32 infections/patient per year in patients treated with FLOLAN. This rate was higher than reported<br />
in patients using chronic indwelling central venous catheters to administer parenteral nutrition, but lower than reported<br />
in oncology patients using these catheters. Malfunctions in the delivery system resulting in an inadvertent bolus<br />
of or a reduc tion in FLOLAN were associated with symptoms related to excess or insuffi cient FLOLAN, respectively (see<br />
ADVERSE REACTIONS: Adverse Events During Chronic Administration). Observed During Clinical Practice: In addition<br />
to adverse reactions reported from clinical trials, the following events have been identifi ed during post-approval use of<br />
FLOLAN. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be<br />
made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting,<br />
or potential causal connection to FLOLAN. Blood and Lymphatic: Anemia, hypersplenism, pancytopenia, splenomegaly.<br />
Endocrine and Metabolic: Hyperthyroidism.<br />
OVERDOSAGE:<br />
Signs and symptoms of excessive doses of FLOLAN during clinical trials are the expected dose-limiting pharmacologic effects<br />
of FLOLAN, including flushing, headache, hypotension, tachycardia, nausea, vomiting, and diarrhea. Treatment will<br />
ordinarily require dose reduction of FLOLAN. One patient with secondary pulmonary hypertension accidentally received 50 mL<br />
of an unspecifi ed concentration of FLOLAN. The patient vomited and became unconscious with an initially unrecordable<br />
blood pressure. FLOLAN was dis continued and the patient regained consciousness within seconds. In clinical practice,<br />
fatal occurrences of hypoxemia, hypotension, and respiratory arrest have been reported following overdosage of FLOLAN.<br />
DOSAGE AND ADMINISTRATION:<br />
Important <strong>No</strong>te: FLOLAN must be reconstituted only with STERILE DILUENT for FLOLAN. Reconstituted solutions of<br />
FLOLAN must not be diluted or administered with other parenteral solutions or medications (see WARNINGS). Dosage:<br />
Continuous chronic infusion of FLOLAN should be administered through a central venous catheter. Temporary peripheral<br />
intravenous infusion may be used until central access is established. Chronic infusion of FLOLAN should be ini tiated at<br />
2 ng/kg/min and increased in increments of 2 ng/kg/min every 15 minutes or longer until dose-limiting pharmaco logic<br />
effects are elicited or until a tolerance limit to the drug is established and further increases in the infusion rate are not<br />
clinically warranted (see Dosage Adjustments). If dose-limiting pharmacologic effects occur, then the infusion rate should<br />
be decreased to an appropriate chronic infusion rate whereby the pharmacologic effects of FLOLAN are tolerated. In clinical<br />
tri als, the most common dose-limiting adverse events were nausea, vomiting, hypotension, sepsis, headache, abdominal<br />
pain, or respiratory disorder (most treatment-limiting adverse events were not serious). If the initial infusion rate of 2 ng/<br />
kg/min is not tolerated, a lower dose that is tolerated by the patient should be identifi ed. In the controlled 12-week trial in<br />
PH/SSD, for example, the dose increased from a mean starting dose of 2.2 ng/kg/min. During the first 7 days of treatment,<br />
the dose was increased daily to a mean dose of 4.1 ng/kg/min on day 7 of treatment. At the end of week 12, the mean dose<br />
was 11.2 ng/kg/min. The mean incremental increase was 2 to 3 ng/kg/min every 3 weeks. Dosage Adjustments: Changes<br />
in the chronic infusion rate should be based on persistence, recurrence, or worsening of the patient’s symptoms of pulmonary<br />
hypertension and the occurrence of adverse events due to excessive doses of FLOLAN. In general, increases in dose<br />
from the initial chronic dose should be expected. Increments in dose should be considered if symptoms of pulmonary hypertension<br />
persist or recur after improving. The infu sion should be increased by 1- to 2-ng/kg/min increments at intervals<br />
suffi cient to allow assessment of clinical response; these intervals should be at least 15 minutes. Following establishment<br />
of a new chronic infusion rate, the patient should be observed, and standing and supine blood pressure and heart rate<br />
monitored for several hours to ensure that the new dose is tolerated. During chronic infusion, the occurrence of dose-limiting<br />
pharmacological events may necessitate a decrease in infusion rate, but the adverse event may occasionally resolve<br />
without dosage adjustment. Dosage decreases should be made gradually in 2-ng/kg/min decrements every 15 minutes or<br />
longer until the dose-limiting effects resolve. Abrupt withdrawal of FLOLAN or sudden large reductions in infusion rates<br />
should be avoided. Except in life-threatening situations (e.g., unconsciousness, collapse, etc.), infusion rates of FLOLAN<br />
should be adjusted only under the direction of a physician. Administration: FLOLAN is administered by continuous intravenous<br />
infusion via a central venous catheter using an ambu latory infusion pump. During initiation of treatment, FLOLAN<br />
may be administered peripherally.<br />
To avoid potential interruptions in drug delivery, the patient should have access to a backup infusion pump and intravenous<br />
infusion sets. A multi-lumen catheter should be considered if other intravenous therapies are routinely administered. To<br />
facilitate extended use at ambient temperatures exceeding 25°C (77°F), a cold pouch with frozen gel packs was used in<br />
clinical trials. Any cold pouch used must be capable of maintaining the temperature of reconstituted FLOLAN between 2°<br />
and 8°C for 12 hours. Reconstitution: FLOLAN is stable only when reconstituted with STERILE DILUENT for FLOLAN.<br />
FLOLAN must not be reconstituted or mixed with any other parenteral medications or solutions prior to or during<br />
administration. Storage and Stability: Unopened vials of FLOLAN are stable until the date indicated on the package<br />
when stored at 15° to 25°C (59° to 77°F) and protected from light in the carton. Unopened vials of STERILE DILUENT for<br />
FLOLAN are stable until the date indicated on the package when stored at 15° to 25°C (59° to 77°F). Prior to use, reconstituted<br />
solutions of FLOLAN must be protected from light and must be refrigerated at 2° to 8°C (36° to 46°F) if not used<br />
immediately. Do not freeze reconstituted solutions of FLOLAN. Discard any reconstituted solution that has been<br />
frozen. Discard any reconstituted solution if it has been refrigerated for more than 48 hours.<br />
©2008, GlaxoSmithKline. All rights reserved. January 2008 FLL:1PI
FOR PATIENTS WITH PULMONARY ARTERIAL HYPERTENSION (PAH) WITH NYHA CLASS II-IV SYMPTOMS<br />
Joanne<br />
REMODULIN patient<br />
Infused with POSSIBILITIES<br />
When initial PAH therapy loses its momentum, think REMODULIN<br />
The first and only prostacyclin available for both SC and IV infusion<br />
Improves symptoms associated with exercise 1,2<br />
Improves hemodynamics 1<br />
May be titrated to effect<br />
Multiple pump options<br />
<strong>No</strong> ice packs<br />
Up to 72 hours (SC) or 48 hours (IV) between reservoir changes<br />
Indications: REMODULIN ® (treprostinil sodium) Injection is indicated for the treatment of pulmonary arterial hypertension in patients with NYHA<br />
Class II-IV symptoms to diminish symptoms associated with exercise. It may be administered as a continuous subcutaneous infusion or continuous<br />
intravenous infusion; however, because of the risks associated with chronic indwelling central venous catheters, including serious blood stream<br />
infections, continuous intravenous infusion should be reserved for patients who are intolerant of the subcutaneous route, or in whom these risks<br />
are considered warranted.<br />
REMODULIN is indicated to diminish the rate of clinical deterioration in patients requiring transition from Flolan ® (epoprostenol sodium) for<br />
Injection; the risks and benefits of each drug should be carefully considered prior to transition.<br />
Important Safety Information: Chronic intravenous infusions of REMODULIN are delivered using an indwelling central venous catheter.<br />
This route is associated with the risk of blood stream infections (BSI) and sepsis, which may be fatal.<br />
REMODULIN is contraindicated in patients with hypersensitivity to REMODULIN, its ingredients, or similar drugs. REMODULIN is a potent vasodilator.<br />
It lowers blood pressure, which may be further lowered by other drugs that also reduce blood pressure. REMODULIN inhibits platelet aggregation<br />
and therefore, may increase the risk of bleeding, particularly in patients on anticoagulants. Abrupt withdrawal or sudden large reductions in dosage<br />
of REMODULIN may result in worsening of PAH symptoms and should be avoided. Caution should be used in patients with hepatic or renal problems.<br />
The most common side effects of REMODULIN included those related to the method of infusion. For subcutaneous infusion, infusion site pain and<br />
infusion site reaction (redness and swelling) occurred in the majority of patients. These symptoms were often severe and could lead to treatment with<br />
narcotics or discontinuation of REMODULIN. For intravenous infusion, line infections, sepsis, arm swelling, paresthesias, hematoma and pain were most<br />
common. General side effects (>5% more than placebo) were diarrhea, jaw pain, vasodilation, and edema.<br />
References: 1. Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil,<br />
a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized,<br />
placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(6):800-804. 2. REMODULIN [package insert].<br />
United Therapeutics Corporation; 2008.<br />
For important safety and other information, please see brief summary of<br />
full prescribing information on the back of this page.<br />
REMODULIN is a registered trademark of United Therapeutics Corporation.<br />
Flolan is a registered trademark of GlaxoSmithKline.<br />
REM_JAd_JUN08v.4<br />
Empowering Prostacyclin
REMODULIN ® (treprostinil sodium) Injection<br />
BRIEF SUMMARY<br />
The following is a brief summary of the full prescribing information on Remodulin<br />
(treprostinil sodium) Injection. Please review the full prescribing information prior<br />
to prescribing Remodulin.<br />
INDICATIONS AND USAGE<br />
Remodulin is indicated for the treatment of pulmonary arterial hypertension in<br />
patients with NYHA Class II-IV symptoms to diminish symptoms associated with<br />
exercise. It may be administered as a continuous subcutaneous (SC) infusion or<br />
continuous intravenous (IV) infusion; however, because of the risks associated<br />
with chronic indwelling central venous catheters, including serious blood stream<br />
infections, continuous IV infusion should be reserved for patients who are<br />
intolerant of the subcutaneous route, or in whom these risks are considered<br />
warranted.<br />
Remodulin is indicated to diminish the rate of clinical deterioration in patients<br />
requiring transition from Flolan ® ; the risks and benefits of each drug should be<br />
carefully considered prior to transition.<br />
DESCRIPTION<br />
Remodulin ® (treprostinil sodium) Injection is a sterile sodium salt supplied in 20 mL<br />
vials in four strengths, containing 1 mg/mL, 2.5 mg/mL, 5 mg/mL or 10 mg/mL of<br />
treprostinil. Each mL also contains 5.3 mg sodium chloride (except for the 10<br />
mg/mL strength which contains 4.0 mg sodium chloride), 3.0 mg metacresol, 6.3<br />
mg sodium citrate, and water for injection.<br />
CONTRAINDICATIONS<br />
Remodulin is contraindicated in patients with known hypersensitivity to the drug or<br />
to structurally related compounds.<br />
WARNINGS<br />
Adverse Events Attributable to the Intravenous Drug Delivery System<br />
Chronic IV infusions of Remodulin are delivered using an indwelling central<br />
venous catheter. This route is associated with the risk of blood stream infections<br />
(BSIs) and sepsis, which may be fatal.<br />
In an open-label study of IV treprostinil (n=47), there were seven catheter-related<br />
line infections during approximately 35 patient years, or about 1 BSI event per 5<br />
years of use. A CDC survey of seven sites that used IV treprostinil for the<br />
treatment of PAH found approximately 1 BSI (defined as any positive blood<br />
culture) event per 3 years of use.<br />
PRECAUTIONS<br />
General<br />
Remodulin should be used only by clinicians experienced in the diagnosis and<br />
treatment of PAH. Remodulin is a potent pulmonary and systemic vasodilator.<br />
Initiation of Remodulin must be performed in a setting with adequate personnel<br />
and equipment for physiological monitoring and emergency care. Therapy with<br />
Remodulin may be used for prolonged periods, and the patient’s ability to<br />
administer Remodulin and care for an infusion system should be carefully<br />
considered. Dose should be increased for lack of improvement in, or worsening of,<br />
symptoms and it should be decreased for excessive pharmacologic effects or for<br />
unacceptable infusion site symptoms. Abrupt withdrawal or sudden large<br />
reductions in dosage of Remodulin may result in worsening of PAH symptoms and<br />
should be avoided.<br />
Information for Patients<br />
Patients receiving Remodulin should be given the following information:<br />
Remodulin is infused continuously through a SC or surgically placed indwelling<br />
central venous catheter, via an infusion pump. Therapy with Remodulin will be<br />
needed for prolonged periods, possibly years, and the patient's ability to accept<br />
and care for a catheter and to use an infusion pump should be carefully<br />
considered. In order to reduce the risk of infection, aseptic technique must be<br />
used in the preparation and administration of Remodulin. Additionally, patients<br />
should be aware that subsequent disease management may require the initiation<br />
of an alternative IV prostacyclin therapy, Flolan ® (epoprostenol sodium).<br />
Drug Interactions<br />
Reduction in blood pressure caused by Remodulin may be exacerbated by drugs<br />
that by themselves alter blood pressure, such as diuretics, antihypertensive<br />
agents, or vasodilators. Since Remodulin inhibits platelet aggregation, there is<br />
also a potential for increased risk of bleeding, particularly among patients<br />
maintained on anticoagulants. During clinical trials, Remodulin was used<br />
concurrently with anticoagulants, diuretics, cardiac glycosides, calcium channel<br />
blockers, analgesics, antipyretics, nonsteroidal anti-inflammatories, opioids,<br />
corticosteroids, and other medications. Remodulin has not been studied in<br />
conjunction with Flolan or Tracleer ® (bosentan).<br />
Effect of Other Drugs on Remodulin<br />
In vivo studies: Acetaminophen - Analgesic doses of acetaminophen, 1000 mg<br />
every 6 hours for seven doses, did not affect the pharmacokinetics of Remodulin,<br />
at a SC infusion rate of 15 ng/kg/min.<br />
Effect of Remodulin on Other Drugs<br />
In vitro studies: Remodulin did not significantly affect the plasma protein binding of<br />
normally observed concentrations of digoxin or warfarin.<br />
In vivo studies: Warfarin - Remodulin does not affect the pharmacokinetics or<br />
pharmacodynamics of warfarin. The pharmacokinetics of R- and S- warfarin and<br />
the INR in healthy subjects given a single 25 mg dose of warfarin were unaffected<br />
by continuous SC Remodulin at an infusion rate of 10 ng/kg/min.<br />
Hepatic and Renal Impairment<br />
Caution should be used in patients with hepatic or renal impairment.<br />
Carcinogenesis, Mutagenesis, Impairment of Fertility<br />
Long-term studies have not been performed to evaluate the carcinogenic potential<br />
of treprostinil. In vitro and in vivo genetic toxicology studies did not demonstrate<br />
any mutagenic or clastogenic effects of treprostinil. Treprostinil sodium did not<br />
affect fertility or mating performance of male or female rats given continuous SC<br />
infusions at rates of up to 450 ng treprostinil/kg/min [about 59 times the<br />
recommended starting human rate of infusion (1.25 ng/kg/min) and about 8 times<br />
the average rate (9.3 ng/kg/min) achieved in clinical trials, on a ng/m2 basis]. In<br />
this study, males were dosed from 10 weeks prior to mating and through the 2-<br />
week mating period. Females were dosed from 2 weeks prior to mating until<br />
gestational day 6.<br />
Pregnancy<br />
Pregnancy Category B - In pregnant rats, continuous SC infusions of treprostinil<br />
sodium during organogenesis and late gestational development, at rates as high<br />
as 900 ng treprostinil/kg/min (about 117 times the starting human rate of infusion,<br />
on a ng/m 2 basis and about 16 times the average rate achieved in clinical trials),<br />
resulted in no evidence of harm to the fetus. In pregnant rabbits, effects of<br />
continuous SC infusions of treprostinil during organogenesis were limited to an<br />
increased incidence of fetal skeletal variations (bilateral full rib or right rudimentary<br />
rib on lumbar 1) associated with maternal toxicity (reduction in body weight and<br />
food consumption) at an infusion rate of 150 ng treprostinil/kg/min (about 41 times<br />
the starting human rate of infusion, on a ng/m 2 basis, and 5 times the average rate<br />
used in clinical trials). In rats, continuous SC infusion of treprostinil from<br />
implantation to the end of lactation, at rates of up to 450 ng treprostinil/kg/min, did<br />
not affect the growth and development of offspring. Because animal reproduction<br />
studies are not always predictive of human response, Remodulin should be used<br />
during pregnancy only if clearly needed.<br />
Labor and delivery<br />
<strong>No</strong> treprostinil sodium treatment-related effects on labor and delivery were seen in<br />
animal studies. The effect of treprostinil sodium on labor and delivery in humans is<br />
unknown.<br />
Nursing mothers<br />
It is not known whether treprostinil is excreted in human milk or absorbed<br />
systemically after ingestion. Because many drugs are excreted in human milk,<br />
caution should be exercised when Remodulin is administered to nursing women.<br />
Pediatric use<br />
Safety and effectiveness in pediatric patients have not been established. Clinical<br />
studies of Remodulin did not include sufficient numbers of patients aged 40 ng/kg/min. Abrupt cessation of infusion should be<br />
avoided (see PRECAUTIONS). Restarting a Remodulin infusion within a few<br />
hours after an interruption can be done using the same dose rate. Interruptions for<br />
longer periods may require the dose of Remodulin to be re-titrated.<br />
Administration<br />
SC Infusion<br />
Remodulin is administered subcutaneously by continuous infusion, via a selfinserted<br />
SC catheter, using an infusion pump designed for SC drug delivery. To<br />
avoid potential interruptions in drug delivery, the patient must have immediate<br />
access to a backup infusion pump and SC infusion sets. The ambulatory infusion<br />
pump used to administer Remodulin should: (1) be small and lightweight, (2) be<br />
adjustable to approximately 0.002 mL/hr, (3) have occlusion/no delivery, low<br />
battery, programming error and motor malfunction alarms, (4) have delivery<br />
accuracy of ±6% or better and (5) be positive pressure driven. The reservoir<br />
should be made of polyvinyl chloride, polypropylene or glass.<br />
For SC infusion, Remodulin is delivered without further dilution at a calculated<br />
SC Infusion Rate (mL/hr) based on a patient’s Dose (ng/kg/min), Weight (kg), and<br />
the Vial Strength (mg/mL) of Remodulin being used. During use, a single<br />
reservoir (syringe) of undiluted Remodulin can be administered up to 72 hours at<br />
37C. The SC Infusion rate is calculated using the following formula:<br />
SC Infusion Rate<br />
(mL/hr)<br />
*Conversion factor of 0.00006 = 60 min/hour x 0.000001 mg/ng<br />
IV Infusion<br />
Remodulin must be diluted with either Sterile Water for Injection or 0.9%<br />
Sodium Chloride Injection and is administered intravenously by continuous<br />
infusion, via a surgically placed indwelling central venous catheter, using an<br />
infusion pump designed for intravenous drug delivery. If clinically necessary, a<br />
temporary peripheral intravenous cannula, preferably placed in a large vein, may<br />
be used for short term administration of Remodulin. Use of a peripheral<br />
intravenous infusion for more than a few hours may be associated with an<br />
increased risk of thrombophlebitis. To avoid potential interruptions in drug<br />
delivery, the patient must have immediate access to a backup infusion pump and<br />
infusion sets. The ambulatory infusion pump used to administer Remodulin<br />
should: (1) be small and lightweight, (2) have occlusion/no delivery, low battery,<br />
programming error and motor malfunction alarms, (3) have delivery accuracy of<br />
±6% or better of the hourly dose, and (4) be positive pressure driven. The<br />
reservoir should be made of polyvinyl chloride, polypropylene or glass. Diluted<br />
Remodulin has been shown to be stable at ambient temperature for up to 48 hours<br />
at concentrations as low as 0.004 mg/mL (4,000 ng/mL). When using an<br />
appropriate infusion pump and reservoir, a predetermined intravenous infusion<br />
rate should first be selected to allow for a desired infusion period length of up to 48<br />
hours between system changeovers. Typical intravenous infusion system<br />
reservoirs have volumes of 50 or 100 mL. With this selected Intravenous Infusion<br />
Rate (mL/hr) and the patient’s Dose (ng/kg/min) and Weight (kg), the Diluted<br />
Intravenous Remodulin Concentration (mg/mL) can be calculated using the<br />
following formula:<br />
Step 1<br />
Diluted IV<br />
Remodulin<br />
Conc. (mg/mL)<br />
The Amount of Remodulin Injection needed to make the required Diluted<br />
Intravenous Remodulin Concentration for the given reservoir size can then be<br />
calculated using the following formula:<br />
Step 2<br />
Amount of<br />
Remodulin<br />
Injection<br />
(mL)<br />
=<br />
Diluted IV<br />
Remodulin Conc.<br />
(mg/mL)<br />
Remodulin Vial<br />
Strength (mg/mL)<br />
x<br />
Total <strong>Vol</strong>ume of<br />
Diluted Remodulin<br />
Solution in<br />
Reservoir<br />
(mL)<br />
The calculated amount of Remodulin Injection is then added to the reservoir along<br />
with the sufficient volume of diluent (Sterile Water for Injection or 0.9% Sodium<br />
Chloride Injection) to achieve the desired total volume in the reservoir.<br />
In patients requiring transition from Flolan:<br />
Transition from Flolan to Remodulin is accomplished by initiating the infusion of<br />
Remodulin and increasing it, while simultaneously reducing the dose of<br />
intravenous Flolan. The transition to Remodulin should take place in a hospital<br />
with constant observation of response (e.g., walk distance and signs and<br />
symptoms of disease progression). During the transition, Remodulin is initiated at<br />
a recommended dose of 10% of the current Flolan dose, and then escalated as<br />
the Flolan dose is decreased (see table below for recommended dose titrations).<br />
Patients are individually titrated to a dose that allows transition from Flolan therapy<br />
to Remodulin while balancing prostacyclin-limiting adverse events. Increases in<br />
the patient’s symptoms of PAH should be first treated with increases in the dose of<br />
Remodulin. Side effects normally associated with prostacyclin and prostacyclin<br />
analogs are to be first treated by decreasing the dose of Flolan.<br />
Recommended Transition Dose Changes<br />
Step Flolan Dose Remodulin Dose<br />
1 Unchanged 10% Starting Flolan Dose<br />
2 80% Starting Flolan Dose 30% Starting Flolan Dose<br />
3 60% Starting Flolan Dose 50% Starting Flolan Dose<br />
4 40% Starting Flolan Dose 70% Starting Flolan Dose<br />
5 20% Starting Flolan Dose 90% Starting Flolan Dose<br />
6 5% Starting Flolan Dose 110% Starting Flolan Dose<br />
7 0<br />
Dose<br />
(ng/kg/min)<br />
110% Starting Flolan Dose +<br />
additional 5-10% increments as<br />
needed<br />
HOW SUPPLIED<br />
Remodulin ® is supplied in 20 mL multi-use vials at concentrations of 1 mg/mL, 2.5<br />
mg/mL, 5 mg/mL, and 10 mg/mL treprostinil, as sterile solutions in water for<br />
injection, individually packaged in a carton. Unopened vials of Remodulin are<br />
stable until the date indicated when stored at 15 to 25 o C (59 to 77 o F). Store at<br />
25 o C (77 o F), with excursions permitted to 15-30 o C (59-86 o F) [see USP Controlled<br />
Room Temperature].<br />
During use, a single reservoir (syringe) of undiluted Remodulin can be<br />
administered up to 72 hours at 37 o C. Diluted Remodulin Solution can be<br />
administered up to 48 hours at 37 o C when diluted to concentrations as low as<br />
0.004 mg/mL in Sterile Water for Injection or 0.9% Sodium Chloride Injection. A<br />
single vial of Remodulin should be used for no more than 30 days after the initial<br />
introduction into the vial.<br />
Parenteral drug products should be inspected visually for particulate matter and<br />
discoloration prior to administration whenever solution and container permit. If<br />
either particulate matter or discoloration is noted, Remodulin should not be<br />
administered.<br />
United Therapeutics Corp., Research Triangle Park, NC 27709<br />
©Copyright 2008 United Therapeutics Corp. All rights reserved.<br />
Rx only<br />
February 2008<br />
Refer to Full Package Insert<br />
for Complete Information<br />
REM_PIBrief_FEB08v.2<br />
=<br />
=<br />
Dose<br />
(ng/kg/min)<br />
x<br />
x<br />
Weight<br />
(kg)<br />
Remodulin Vial Strength<br />
(mg/mL)<br />
Weight<br />
(kg)<br />
IV Infusion Rate (mL/hr)<br />
x 0.00006*<br />
x 0.00006
Announcing the Programs of the<br />
new <strong>PHA</strong> Medical Education Fund<br />
Thirty-City Medical Education Tour<br />
<strong>PHA</strong>’s Thirty-City Medical Education Tour will visit cities<br />
remote from larger PH centers across the United States,<br />
aiming to present information on the diagnosis and management<br />
of PAH to physicians and other health professionals<br />
that do not have regular access to comprehensive sessions<br />
on pulmonary hypertension. This will encourage collaborative<br />
peer team building and provide more resources for referrals<br />
to specialized centers. Programs will begin in the spring of<br />
2009. Physician CME and nursing CEU credits will be available.<br />
For information on the specifics of this program and<br />
the cities where events will take place, please email<br />
30CityTour@<strong>PHA</strong>ssociation.org.<br />
Committee Chair<br />
Darren Taichman, MD<br />
Penn-Presbyterian Medical Center<br />
Philadelphia, Pennsylvania<br />
Committee Advisor<br />
Michael McGoon, MD<br />
Mayo Clinic<br />
Rochester, Minnesota<br />
Preceptorship Program<br />
The new Preceptorship Program will facilitate direct<br />
education and training of medical professionals, focusing on<br />
cardiologists, pulmonologists, rheumatologists and primary<br />
care physicians that have PAH patients. This program is<br />
aimed at improving connections between referring physicians<br />
and specialists. Led by experienced pulmonary hypertension<br />
specialists in clinical settings, this program will offer physician<br />
CME and nursing CEU credits for participants. For more<br />
information about the Preceptorship Program, please<br />
email Preceptorship@<strong>PHA</strong>ssociation.org.<br />
Committee Chair<br />
Todd Bull, MD<br />
<strong>University</strong> of Colorado<br />
Health Sciences Center<br />
Denver, Colorado<br />
Committee Advisor<br />
Vallerie McLaughlin, MD<br />
<strong>University</strong> of Michigan<br />
Ann Arbor, Michigan<br />
<strong>PHA</strong> <strong>Online</strong> <strong>University</strong><br />
The <strong>PHA</strong> <strong>Online</strong> <strong>University</strong> will be a focused information<br />
resource for medical education in pulmonary hypertension<br />
and will address all levels of expertise and medical interest.<br />
The website will be launched in the spring of 2009, and<br />
aims to be the most comprehensive online resource on<br />
pulmonary hypertension for primary care physicians,<br />
specialists and allied health professionals. Physician CME<br />
and Nursing CEU components will be built into the website.<br />
For information on <strong>PHA</strong> <strong>Online</strong> <strong>University</strong>, please email<br />
<strong>PHA</strong><strong>Online</strong>Univ@<strong>PHA</strong>ssociation.org.<br />
Committee Chair<br />
Robert Frantz, MD<br />
Mayo Clinic<br />
Rochester, Minnesota<br />
<strong>PHA</strong> on the Road: PH Patients and<br />
Families Education Forum<br />
The new Medical Education Program for Patients, <strong>PHA</strong><br />
on the Road, will be visiting Southern California, Southeast<br />
Michigan, Central Florida and New England in the spring<br />
of 2009. The goal of these one-day education seminars is<br />
to present information on the mechanisms, diagnosis and<br />
treatment of PAH to patients and their family members in<br />
a face-to-face setting. For information about <strong>PHA</strong> on the<br />
Road, please email ontheroad@<strong>PHA</strong>ssociation.org.<br />
Committee Chair<br />
Charles Burger, MD<br />
Mayo Clinic Florida<br />
Jacksonville, Florida<br />
Committee Advisor<br />
David Badesch, MD<br />
<strong>University</strong> of Colorado<br />
Health Sciences Center<br />
Aurora, Colorado<br />
The programs of the <strong>PHA</strong> Medical Education<br />
Fund are made possible through unrestricted<br />
educational grants from our sponsors:<br />
PLATINUM:<br />
GOLD:<br />
SILVER:
Advances in Pulmonary Hypertension CME Section<br />
Program Overview<br />
Pulmonary arterial hypertension (PAH), an incurable<br />
disease, is characterized by medial hypertrophy, intimal<br />
fibrosis, and in situ thrombi in small muscular pulmonary<br />
arteries. PAH was considered a rapidly fatal illness with<br />
a median survival of 2.8 years in the 1980s when no<br />
evidence-based therapies were available. Since then<br />
the treatment of this disease has made tremendous<br />
advances, and the last 10 years have seen the discovery<br />
of new medications that have positively influenced the<br />
prognosis and survival of patients with PAH.<br />
This self-study activity is based on 5 articles that review<br />
the latest information on new treatments, combinations<br />
of therapies, and data from phase 1 and 2 clinical trials.<br />
This activity is jointly sponsored by the <strong>University</strong> of<br />
Michigan Medical School and the Pulmonary Hypertension<br />
Association and supported by an unrestricted<br />
education grant from Actelion Pharmaceuticals US, Inc,<br />
Encysive Pharmaceuticals, Inc, Gilead Sciences, Inc,<br />
Pfizer, Inc, and United Therapeutics Corporation.<br />
Target Audience<br />
This self-study activity is appropriate for cardiologists,<br />
pulmonologists, rheumatologists, and other physicians<br />
who treat patients with pulmonary hypertension.<br />
Learning Objectives<br />
Upon completion of this activity participants will be<br />
able to:<br />
1. Review the various animal models used in PH<br />
research to date<br />
2. Compare and contrast the different pathological<br />
findings in animal models of PH<br />
3. Define the metabolic syndrome<br />
4. Define “diabetic cardiomyopathy”<br />
5. Review the effects of metabolic syndrome on<br />
energy production in cardiac myocytes<br />
6. Identify novel hemodynamic measurements that<br />
can be made during right heart catheterization<br />
Self-Assessment Examination<br />
See pages 351 and 352 for self-assessment questions,<br />
answer key, and evaluation form.<br />
Faculty<br />
Karen Fagan, MD<br />
Chief, Division of Pulmonary and<br />
Critical Care Medicine<br />
<strong>University</strong> of South Alabama<br />
Mobile, Alabama<br />
Contributing Authors<br />
Heiko Bugger, MD, PhD<br />
Division of Endocrinology, Metabolism and<br />
Diabetes<br />
Program in Human Molecular Biology and<br />
Genetics<br />
<strong>University</strong> of Utah School of Medicine<br />
E. Dale Abel, MD, PhD<br />
Division of Endocrinology, Metabolism and<br />
Diabetes<br />
Program in Human Molecular Biology and<br />
Genetics<br />
<strong>University</strong> of Utah School of Medicine<br />
Paul M. Hassoun, MD<br />
Professor of Medicine and Director of the<br />
Pulmonary Hypertension Program<br />
Division of Pulmonary and Critical Care Medicine<br />
Johns Hopkins <strong>University</strong>, School of Medicine<br />
Kurt Stenmark, MD<br />
Department of Pediatrics<br />
Developmental Lung Biology Laboratory<br />
<strong>University</strong> of Colorado at Denver and<br />
Health Sciences Center<br />
Hunter C. Champion, MD, PhD<br />
Pulmonary Hypertension Program and<br />
Division of Cardiology<br />
Department of Medicine<br />
Johns Hopkins Medical Institutions<br />
Ivan F. McMurtry, PhD<br />
Departments of Pharmacology and<br />
Medicine and Center for Lung Biology<br />
<strong>University</strong> of South Alabama<br />
Agenda<br />
The Metabolic Syndrome and Cardiac Function<br />
Heiko Bugger, MD, PhD and<br />
E. Dale Abel, MD, PhD<br />
National Heart Lung and Blood Institute Hopkins<br />
Specialized Center in Clinical Oriented Research<br />
(SCCOR): Molecular Determinants of Pulmonary<br />
Arterial Hypertension<br />
Paul M. Hassoun, MD<br />
Specialized Center in Clinical Oriented Research<br />
(SCCOR) Update: Mechanisms and Treatment of<br />
Long Vascular Disease in Infants and Children<br />
Kurt Stenmark, MD<br />
Getting More From Right Heart Catheterization:<br />
A Focus on the Right Ventricle<br />
Hunter C. Champion, MD, PhD<br />
Animal Models of Human Severe PAH<br />
Ivan F. McMurtry, PhD<br />
330 Advances in Pulmonary Hypertension
Accreditation Statement<br />
This activity has been planned and implemented in<br />
accordance with the Essential Areas and Policies of the<br />
Accreditation Council for Continuing Medical Education<br />
(ACCME) through the joint sponsorship of the <strong>University</strong><br />
of Michigan Medical School and the Pulmonary Hypertension<br />
Association. The <strong>University</strong> of Michigan is accredited<br />
by the ACCME to provide continuing medical education<br />
to physicians.<br />
Credit Designation<br />
The <strong>University</strong> of Michigan Medical School designates<br />
this activity for a maximum of 2.0 AMA PRA Category 1<br />
Credits. Physicians should claim credit commensurate<br />
with the extent of their participation in the activity.<br />
Instructions for Earning Credit<br />
This activity is a self-study program; a self-assessment<br />
examination is included on page 351 to help physicians<br />
review important points. A form is also included on page<br />
352 for physicians to evaluate the CME activity. Completion<br />
of this activity involves reading the journal and<br />
completing the self-assessment examination and evaluation<br />
form, which may take up to 2 hours. Credits for this<br />
self-study program are available from February 1 2009<br />
through February 1 2010. There is no fee for this<br />
program.<br />
Please note that this self-study program may also be<br />
viewed online at: http://www.cme.med.umich.edu<br />
<strong>University</strong> of Michigan Privacy Statement<br />
http://www.cme.med.umich.edu/privacy.asp<br />
Sponsorship<br />
This CME self-study program is jointly sponsored by<br />
the <strong>University</strong> of Michigan Medical School and the<br />
Pulmonary Hypertension Association.<br />
Support<br />
This CME self-study program is supported by an educational<br />
grant from Actelion Pharmaceuticals US, Inc.,<br />
Encysive Pharmaceuticals, Inc., Gilead Sciences, Inc.,<br />
Pfizer, Inc., and United Therapeutics Corporation.<br />
Oversite and Accreditation<br />
Arlene Bradford, BA<br />
Assistant Director<br />
Office of CME<br />
<strong>University</strong> of Michigan Medical School<br />
Disclosures<br />
The Accreditation Council for Continuing Medical Education<br />
and the Association of American Colleges has standards<br />
and guidelines to ensure that individuals participating<br />
in CME activities are aware of relationships between<br />
authors and commercial companies that could potentially<br />
affect the information presented. To be disclosed<br />
to participants are all personal financial relationships with<br />
a commercial interest whose products are relevant to the<br />
content of this CME activity. The <strong>University</strong> of Michigan<br />
Medical School follows these national policies to ensure<br />
balance, independence, objectivity, and scientific rigor in<br />
all its CME activities. Each author was asked to complete<br />
a disclosure information form for this activity. Disclosures<br />
are reported below.<br />
Karen Fagan, MD, in the past 12 months has had no<br />
relevant personal financial relationship with regard to the<br />
content of this CME activity. Dr. Fagan is on the Speaker<br />
Panel, Advisory Board and Research Review Committee<br />
of Gilead.<br />
Heiko Bugger, MD, PhD, in the past 12 months has had<br />
no relevant personal financial relationship with regard to<br />
the content of this CME activity.<br />
E. Dale Abel, MD, PhD, in the past 12 months has had no<br />
relevant personal financial relationship with regard to the<br />
content of this CME activity.<br />
Paul M. Hassoun, in the past 12 months has had no<br />
relevant personal financial relationship with regard to the<br />
content of this CME activity.<br />
Kurt Stenmark, MD, in the past 12 months has had no<br />
relevant personal financial relationship with regard to the<br />
content of this CME activity.<br />
Hunter C. Champion, MD,PhD, in the past 12 months has<br />
had no relevant personal financial relationship with regard<br />
to the content of this CME activity. He has served as a<br />
Consultant for Actelion, Gilead, Pfizer, and United<br />
Therapeutics.<br />
Ivan F. McMurtry, PhD, in the past 12 months has had no<br />
relevant personal financial relationship with regard to the<br />
content of this CME activity.<br />
Arlene Bradford, BA, has no relevant personal financial<br />
relationships to disclose.<br />
CME Reviewer<br />
Kevin M. Chan, MD<br />
Assistant Professor of Medicine<br />
Division of Pulmonary and Critical Care Medicine<br />
<strong>University</strong> of Michigan Health Systems<br />
Ann Arbor, Michigan<br />
Dr Chan has no relevant personal financial relationships<br />
to disclose.<br />
Advances in Pulmonary Hypertension 331
Continuing Medical Education Section<br />
The Metabolic Syndrome and Cardiac Function<br />
Heiko Bugger, MD, PhD and E. Dale Abel, MD, PhD<br />
Division of Endocrinology, Metabolism and Diabetes<br />
Program in Human Molecular Biology and Genetics<br />
<strong>University</strong> of Utah School of Medicine<br />
Salt Lake City, Utah<br />
Heiko Bugger, MD<br />
E. Dale Abel, MD<br />
The metabolic syndrome includes obesity, insulin resistance, dyslipidemia,<br />
and type 2 diabetes mellitus. It increases the risk of developing<br />
cardiovascular diseases, including heart failure. Evidence<br />
is emerging that changes in energy metabolism might contribute<br />
to the development of cardiac myocyte contractile dysfunction.<br />
The focus of our laboratory is in understanding the potential molecular<br />
mechanisms for these abnormalities.<br />
Over 40% of US citizens older than 60 years have metabolic<br />
syndrome and the prevalence of the metabolic syndrome parallels<br />
the global epidemic of obesity and diabetes. 1 What is unknown<br />
is the prevalence of the metabolic syndrome and/or type 2<br />
diabetes in patients with pulmonary hypertension. More importantly,<br />
the impact that these comorbidities may have on right ventricular<br />
performance and patient outcomes is not known. The<br />
objective of this article is to review the cardiac effects of the metabolic<br />
syndrome and highlight possible areas for investigation in determinants<br />
of right ventricular dysfunction.<br />
Metabolic Alterations<br />
Obesity, insulin resistance and diabetes increase the risk of developing<br />
cardiovascular disease. 2-4 Many believe that the major<br />
determinant of cardiovascular complications in the metabolic syndrome<br />
is coronary artery disease. Our work suggests that the metabolic<br />
alterations that occur in obesity and type 2 diabetes can<br />
also affect cardiac structure and function independently of hypertension<br />
or coronary artery disease. In addition, after adjusting<br />
for age, blood pressure, weight, cholesterol, and coronary artery<br />
disease, obesity is associated with an increased risk of heart failure.<br />
3,5,6 This “diabetic cardiomyopathy” is defined as ventricular<br />
Address for reprints and other correspondence: E. Dale Abel, MD, Chief, Division<br />
of Endocrinology and Professor of Medicine and Biochemistry, Division<br />
of Endocrinology, Metabolism and Diabetes, Program in Human Molecular<br />
Biology and Genetics, <strong>University</strong> of Utah School of Medicine, 15 N. 2030<br />
East, Rm 3110, Salt Lake City, UT 84112; email: stacis@hmbg.utah.edu.<br />
Acknowledgments—Studies in the Abel laboratory are supported by research<br />
grants from the National Institutes of Health: UO1HL70525, UO1 HL087947<br />
(Animal Models of Diabetes Complications Consortium [AMDCC)]); RO1<br />
HL70070 and RO1 HL73167, the American Heart Association, and the Juvenile<br />
Diabetes Research Foundation. Dr Bugger is supported by a postdoctoral<br />
fellowship from the German Research Foundation.<br />
systolic or diastolic dysfunction occurring in diabetic patients in<br />
the absence of coronary artery disease and hypertension. 7-9 Several<br />
studies have identified the presence of lipid material in the<br />
hearts of patients who are obese or have type 2 diabetes who have<br />
had nonischemic heart failure. 10,11 The transcriptional profile of<br />
these lipid-laden hearts is similar to that of the Zucker diabetic rat<br />
(ZDF), an animal model of lipotoxicity and contractile dysfunction,<br />
which suggests that dysregulation of fatty acid metabolism<br />
in failing human hearts may contribute to contractile dysfunction.<br />
Most mechanistic insights into obesity-related cardiomyopathy<br />
and diabetic cardiomyopathy have come from rodent studies. The<br />
most widely investigated models are db/db mice (leptin receptor<br />
mutation), ob/ob mice (leptin deficiency), and ZDF rats (leptin receptor<br />
mutation). All of these models have obesity, insulin resistance,<br />
and hyperglycemia in common, although to varying degrees<br />
in each model. 12,13 These animals do not develop atherosclerosis,<br />
which allows an evaluation of the effects of obesity, insulin resistance,<br />
and type 2 diabetes in the heart that are independent of<br />
coronary artery disease. 13,14 Each of these animal models is associated<br />
with evidence of contractile dysfunction, both systolic and<br />
diastolic, which further supports the existence of an obesity-related<br />
and/or diabetic cardiomyopathy. 15-24<br />
Pathophysiology<br />
Patients with type 2 diabetes have decreased whole-body aerobic<br />
capacity that may be related to decreased expression of mitochondrial<br />
proteins in skeletal muscle. 25-28 Mitochondrial function<br />
and morphology are also abnormal in prediabetic and diabetic<br />
states and include a reduction in overall mitochondrial size and<br />
content and a 30% reduction in ATP synthesis. 29-32 What is not<br />
clear is whether these skeletal muscle mitochondrial abnormalities<br />
represent a genetic predisposition to the metabolic syndrome<br />
or acquired defects. 33,34<br />
Cardiac muscle mitochondria have been less well studied. A<br />
few indirect studies suggest that myocardial mitochondrial function<br />
is altered in obesity and diabetes as evidenced by increased<br />
oxygen consumption and reduced cardiac efficiency. 35 Which are,<br />
in turn, associated with increased myocardial fatty acid use and<br />
impaired glucose tolerance. More direct evidence for cardiac mi-<br />
332 Advances in Pulmonary Hypertension
Figure. Model for Synergistic Effects of Insulin Resistance and FA Excess in Precipitating Mitochondrial<br />
Dysfunction in Hearts. FA, fatty acids; ROS, reactive oxygen species; UCP, uncoupling protein; ANT, adenine<br />
nucleotide translocase; ATP, adenosine triphosphate; ADP, adenosine diphosphate.<br />
tochondrial dysfunction in patients with type 2 diabetes has come<br />
from studies using 31 P nuclear magnetic resonance (NMR) spectroscopy.<br />
Findings from these studies suggest that patients with<br />
type 2 diabetes have reduced cardiac phosphocreatine/adenosine<br />
triphosphate (ATP) ratios, and impaired high-energy phosphate<br />
metabolism and a cardiac energy deficit. 36,37 Phosphocreatine/ATP<br />
ratios are also decreased in failing hearts of other etiologies, which<br />
are associated with mitochondrial dysfunction. 38-40 In addition,<br />
plasma-free fatty acid concentrations were found to correlate negatively<br />
with phosphocreatine/ATP ratios in patients with diabetes. 37<br />
This may be due to increased expression of uncoupling proteins<br />
(UCPs) that reduce the efficiency of ATP production and lead to<br />
reduced phosphocreatine/ATP ratios. Increased lipid deposition<br />
has been found in diabetic cardiomyopathy and may exceed mitochondrial<br />
fatty acid oxidative capacity. This results in increased<br />
lipid storage instead of oxidation and lipotoxic effect. 11<br />
In contrast with human studies, mitochondrial function has<br />
been directly investigated in several animal models of metabolic<br />
syndrome. Mitochondrial dysfunction is present in the type 2 diabetic<br />
rodent heart as demonstrated by reduced mitochondrial<br />
respiration and ATP synthesis. 41-43 Mitochondrial structural defects<br />
and abnormal mitochondrial proliferation also occur in ob/ob<br />
mice. 44-46<br />
The heart depends on continuous oxidative metabolism for<br />
ATP generation to maintain contractile function. Mitochondria account<br />
for approximately 40% of cardiomyocyte volume. The normal<br />
heart generates ATP mainly from the mitochondrial oxidation<br />
of fatty acids (60% to 70% of ATP generated) and to a lesser extent<br />
from glucose, lactate, and other substrates (30% to 40%). 19,20,23<br />
The increased myocardial fatty acid oxidative capacity in obesity<br />
and diabetes are mediated, in part, by increased activity of peroxisome<br />
proliferator-activated receptors (PPARs) (in particular<br />
PPARα). PPARα has been shown to be a central regulator of fatty<br />
acid oxidation in the heart by increasing the expression of genes<br />
involved in virtually every step of cardiac fatty acid utilization. 47<br />
Conversely PPARα reduces the expression of genes that regulate<br />
glucose use and thereby contribute to reduced glucose oxidation.<br />
Mice with cardiac overexpression of<br />
PPARα mimicked the metabolic phenotype<br />
of the diabetic heart, which implicates<br />
PPARα in the regulation of cardiac<br />
metabolism in the diabetic heart.<br />
Theoretical calculations of the yield<br />
of ATP per oxygen atom consumed show<br />
that fatty acids are a less efficient fuel<br />
when compared with glucose. 48 It is calculated<br />
that shifting from 100% palmitate<br />
to 100% glucose would increase the<br />
ATP yield per molecule of oxygen consumed<br />
by 12% to 14%. Thus, increased<br />
fatty acid use in the diabetic heart may<br />
be energetically detrimental because of<br />
the higher oxygen cost to produce ATP.<br />
The higher oxygen cost and the decrease<br />
in cardiac efficiency may contribute to<br />
the development of contractile dysfunction<br />
in the metabolic syndrome. Cardiac<br />
energy depletion may become even more<br />
pronounced by the coexistence of hypertension<br />
(a common comorbidity in<br />
the metabolic syndrome), which increases<br />
the energy demand for the heart. In addition, these mechanisms<br />
may also contribute to the increased susceptibility to<br />
ischemic damage and poorer outcomes after myocardial infarction.<br />
The mechanisms for increased myocardial oxygen consumption<br />
and decreased cardiac efficiency are incompletely understood.<br />
Our findings suggest increased mitochondrial uncoupling<br />
as one underlying mechanism. 43,49 Mitochondrial uncoupling increases<br />
oxygen consumption without proportionately increasing<br />
mitochondrial ATP production. The energy deficit that results may<br />
explain the lack of increase in cardiac contractile function and<br />
reduced cardiac efficiency.<br />
One of the mechanisms leading to cardiac mitochondrial uncoupling<br />
in type 2 diabetes may be the increased expression of<br />
UCPs (Figure). These proteins allow the H+ generated from the<br />
transfer of electrons from oxygen to re-enter the mitochondrial intermembrane<br />
space without generation of ATP from adenosine<br />
diphosphate (ADP) thus uncoupling oxygen consumption from ATP<br />
generation. Several UCPs have been identified. 50-59 Both UCP2<br />
and UCP3 are expressed in the heart, but their roles are still unclear.<br />
60,61 Circulating free fatty acid levels correlate with the expression<br />
of UCP2 and UCP3 in the human heart, which suggests<br />
that plasma free fatty acid concentrations may regulate cardiac<br />
UCP expression, possibly through activation of PPARα-response elements<br />
in the UCP promoter regions. 61-65<br />
Proton leak via the adenine nucleotide translocator (ANT) may<br />
also lead to uncoupling (Figure). This protein was shown to mediate<br />
uncoupling by fatty acids and to lower mitochondrial membrane<br />
potential in heart and skeletal muscle. 66,67 Studies that used<br />
inhibitors of ANT suggest that the large part of mitochondrial uncoupling<br />
was mediated by UCPs, but that a small part of proton<br />
leak was also mediated by ANT activity. 49<br />
Another mechanism that may lead to decreased cardiac contractility<br />
is through generation of reactive oxygen species (ROS).<br />
Mitochondria are the principal source of ROS in cells. <strong>No</strong>rmally,<br />
electrons are funneled through the redox carriers of the respiratory<br />
chain to molecular oxygen reducing O 2<br />
to water. Even during nor-<br />
Advances in Pulmonary Hypertension 333
mal metabolism, some electrons leak from the respiratory chain,<br />
which results in the generation of reactive incompletely reduced<br />
forms of oxygen, such as superoxide and hydroxyl anions. Increased<br />
electron delivery from increased glucose oxidation or increased<br />
fatty acid oxidation have been shown to increase<br />
mitochondrial ROS generation. 68,69<br />
ROS can severely harm the cell through oxidation of proteins,<br />
DNA (including mitochondrial DNA), and nitrosylation of proteins<br />
(through generation of reactive nitrogen species) and lead to improper<br />
protein function (Figure). Oxidative stress is widely accepted<br />
as a key player in the development and progression of<br />
diabetes and its complications, including cardiac pathologies. 70-74<br />
In diabetes, ROS may be predominantly derived from mitochondria<br />
as opposed to cytosolic origins. 68,75,76 Mitochondria are not<br />
only the origin, but also the target of oxidative stress. In addition<br />
to the direct effects on proteins and DNA, ROS can also induce<br />
mitochondrial uncoupling. 49<br />
Most studies that investigate the effect of ROS on mitochondrial<br />
function in diabetic hearts have been performed in type 1 diabetic<br />
models. In these animal models, cardiac mitochondrial<br />
respiratory dysfunction has been demonstrated and, in some studies,<br />
improved antioxidant defense was able to at least partially, if<br />
not completely, restore mitochondrial respiratory function. 77-80 The<br />
possibility that the mechanisms by which ROS causes mitochondrial<br />
damage are similar in type 2 diabetes and is supported by<br />
several similar observations in type 2 diabetic models. 49,78,81-83<br />
A recent study suggests that mitochondrial ROS overproduction<br />
may play a greater role in impairing mitochondrial energetics<br />
in models of insulin resistance and obesity versus models of insulin<br />
deficiency and type 1 diabetes. 84 It appears likely that ROS<br />
plays a central role in impaired mitochondrial energy metabolism<br />
by participating in mitochondrial uncoupling (in type 2 diabetes<br />
and cardiac efficiency) thus directly damaging mitochondrial proteins.<br />
Both mechanisms probably contribute to a deficit in energy<br />
reserve and contribute to the development of contractile dysfunction.<br />
Cardiac performance also depends on the influx of Ca2+. It exposes<br />
active sites on actin, which interact with myosin crossbridges<br />
in an energy-requiring reaction. At the end of the contraction,<br />
Ca2+ is rapidly removed from the cytosol. Ca2+ exchange<br />
between these subcellular compartments is believed to provide a<br />
mechanism for matching energy production to energy demand<br />
under physiological conditions or increased workload and is<br />
termed the “parallel activation model.” 85<br />
Although some Ca2+ is exported via the sarcolemmal membrane,<br />
the bulk of Ca2+ is resequestered in the sarcoplasmic reticulum<br />
by the activity of sarcoplasmic/endoplasmic reticulum<br />
Ca2+-ATPase 2a (SERCA2a). 86-88 Contractile dysfunction in the<br />
diabetic heart has been proposed to be the consequence of abnormalities<br />
in sarcoplasmic reticulum Ca2+ handling and has<br />
been specifically attributed to the decreased expression of<br />
SERCA2a. 89-93<br />
It has recently been demonstrated that mitochondrial biogenesis<br />
occurs in hearts of obese and insulin resistant animals. 44,45<br />
However, this was not associated with increased mitochondrial<br />
respiration or ATP generation. We have also observed increased<br />
mitochondrial density and DNA content in ob/ob and db/db mice<br />
despite impaired ADP stimulated respiration and ATP synthesis.<br />
43,44,49 These observations raise the question whether mitochondrial<br />
biogenesis is adaptive or maladaptive in the metabolic<br />
syndrome.<br />
Given that animal models of the metabolic syndrome exhibit<br />
insulin resistance the question arises whether cardiac insulin<br />
resistance may contribute to the development of contractile<br />
dysfunction. Since the animal models are characterized by systemic<br />
metabolic alterations, evaluation of the contribution of insulin<br />
resistance to cardiomyocyte contractile dysfunction is<br />
challenging. To approach this problem, we generated mice with a<br />
deletion of the insulin receptor (CIRKO mice) restricted to the<br />
cardiomyocyte. 94<br />
CIRKO mice have reduced insulin-stimulated glucose uptake<br />
and also have a modest decrease in contractile function, thereby<br />
insulin resistance may be a contributing factor in contractile dysfunction<br />
in the metabolic syndrome. This may be caused by decreased<br />
mitochondrial gene expression, which limits oxidative<br />
capacity and impairs mitochondrial energetics and contractile<br />
function in CIRKO mice. If this is correct, then CIRKO hearts may<br />
be more susceptible to injury when subjected to increased energy<br />
demands<br />
CIRKO mice subjected to pressure overload through transverse<br />
aortic banding or following chronic β-adrenergic stimulation<br />
resulted in worse left ventricular dysfunction, left ventricular<br />
dilation, and interstitial fibrosis compared to controls. 95,96 These<br />
findings support the notion that insulin resistance may play a<br />
role in the development of contractile dysfunction in the metabolic<br />
syndrome, and impaired myocardial mitochondrial oxidative<br />
capacity due to reduced insulin action could be an underlying<br />
mechanism.<br />
Conclusion<br />
It is probable that no one single mechanism, but rather the combination<br />
of several mechanisms, leads to cardiac dysfunction in<br />
the metabolic syndrome. We propose that mitochondrial dysfunction<br />
compromises cardiac ATP generation and leads to contractile<br />
dysfunction. <strong>No</strong>vel treatments that target these abnormalities<br />
might lead to new therapeutic avenues for the prevention<br />
of cardiac dysfunction. What is not known is if the mechanisms<br />
of cardiac dysfunction outlined above can be extrapolated<br />
to the right ventricle.<br />
References<br />
1. Zimmet P, Alberti K G, Shaw J. Global and societal implications of the diabetes<br />
epidemic. Nature. 2001;414:782-787.<br />
2. DECODE. Glucose tolerance and cardiovascular mortality: comparison of<br />
fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397-405.<br />
3. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure.<br />
N Engl J Med. 2002;347:305-313.<br />
4. Stamler., Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors,<br />
and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor<br />
Intervention Trial. Diabetes Care. 1993;16:434-444.<br />
5. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure:<br />
the Framingham Study. J Am Coll Cardiol. 1993;22:6A-13A.<br />
6. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham<br />
study. J Am Med Assoc. 1979;41:2035-2038.<br />
7. Regan TJ, Lyons MM, Ahmed SS, et al. Evidence for cardiomyopathy in familial<br />
diabetes mellitus. J Clin Invest. 1977;60:884-899.<br />
8. Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949-2951.<br />
9. Fein FS. Diabetic cardiomyopathy. Diabetes Care. 1990;13:1169-1179.<br />
10. Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides<br />
and systolic function in humans: in vivo evaluation by localized proton spectroscopy<br />
and cardiac imaging. Magn Reson Med. 2003;49:417-423.<br />
11. Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation<br />
in the failing human heart resembles the lipotoxic rat heart. FASEB J.<br />
2004;18:1692-1700.<br />
12. Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic<br />
hearts. Biochim Biophys Acta. 2005;1734:112-126.<br />
334 Advances in Pulmonary Hypertension
13. Russell JC, Proctor SD. Small animal models of cardiovascular disease:<br />
tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis.<br />
Cardiovasc Pathol. 2006;15:318-330.<br />
14. Hsueh W, Abel ED, Breslow JL, et al. Recipes for creating animal models<br />
of diabetic cardiovascular disease. Circ Res. 2007;100:1415-1427.<br />
15. Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of<br />
cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J<br />
Physiol Heart Circ Physiol. 2002;283:H976-H982.<br />
16. Aasum E, Belke DD, Severson DL, et al. Cardiac function and metabolism<br />
in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-apha<br />
activator. Am J Physiol Heart Circ Physiol. 2002;283:H949-H957.<br />
17. Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes<br />
cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol<br />
Endocrinol Metab. 2000;279:E1104-E1113.<br />
18. Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes<br />
in metabolism, contractile function, and ischemic sensitivity in hearts from<br />
db/db mice. Diabetes. 2003;52:434-441.<br />
19. Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and<br />
altered substrate metabolism precedes the onset of hyperglycemia and contractile<br />
dysfunction in two mouse models of insulin resistance and obesity.<br />
Endocrinology. 2005;146:5341-5349.<br />
20. Mazumder PK, O’Neill BT, Roberts MW, et all. Impaired cardiac efficiency<br />
and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes.<br />
2004;53:2366-2374.<br />
21. Yue P, Arai T, Terashima M, et al. Magnetic resonance imaging of progressive<br />
cardiomyopathic changes in the db/db mouse. Am J Physiol Heart<br />
Circ Physiol. 2007;292:H2106-H2118.<br />
22. El-Omar MM, Yang ZK, Phillips AO, Shah AM. Cardiac dysfunction in the<br />
Goto-Kakizaki rat: a model of type II diabetes mellitus. Basic Res Cardiol.<br />
2004;99:133-141.<br />
23. Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate<br />
utilization on cardiac function in isolated hearts from Zucker diabetic<br />
fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102-H2110.<br />
24. Young ME, Guthrie PH, Razeghi P, et al. Impaired long-chain fatty acid oxidation<br />
and contractile dysfunction in the obese Zucker rat heart. Diabetes.<br />
2002;51:2587-2595/<br />
25. Schneider SH, Amorosa LF, Khachadurian AK, Ruderman NB. Studies on<br />
the mechanism of improved glucose control during regular exercise in type 2<br />
(non-insulin-dependent) diabetes. Diabetologia. 1984;26:355-360.<br />
26. Wisløff U, Najjar SM, Ellingsen O, et al. Cardiovascular risk factors emerge<br />
after artificial selection for low aerobic capacity. Science. 2005;307:418-<br />
420.<br />
27. Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1a-responsive genes involved<br />
in oxidative phosphorylation are coordinately downregulated in human<br />
diabetes. Nat Genet. 2003;34:267-273.<br />
28. Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of<br />
oxidative metabolism in humans with insulin resistance and diabetes: Potential<br />
role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466-8471.<br />
29. Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and<br />
increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring<br />
of type 2 diabetic parents. J Clin Invest. 2005;115:3587-3593.<br />
30. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman, GI. Impaired mitochondrial<br />
activity in the insulin-resistant offspring of patients with type 2 diabetes.<br />
N Engl J Med. 2004;350:664-671.<br />
31. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria<br />
in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944-2950.<br />
32. Choo HJ, Kim JH, Kwon OB, et al. Mitochondria are impaired in the<br />
adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784-791.<br />
33. Crunkhorn S, Dearie F, Mantzoros C, et al. PGC-1 expression is reduced<br />
in obesity: Potential pathogenic role of saturated fatty acids and p38 map kinase<br />
activation. J Biol Chem. 2007:282:15439-15450.<br />
34. Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates<br />
genes required for mitochondrial oxidative phosphorylation in skeletal<br />
muscle. Diabetes. 2005;54:1926-1933.<br />
35. Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin<br />
resistance on myocardial substrate metabolism and efficiency in young<br />
women. Circulation. 2004;109:2191-2196.<br />
36. Diamant M, Lamb HJ, Groeneveld Y, et al. Diastolic dysfunction is associated<br />
with altered myocardial metabolism in asymptomatic normotensive patients<br />
with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol.<br />
2003;42:328-335.<br />
37. Scheuermann-Freestone M, Madsen PL, Manners D, et al. Abnormal cardiac<br />
and skeletal muscle energy metabolism in patients with type 2 diabetes.<br />
Circulation. 2003;107:3040-3046.<br />
38 .Casademont J, Miro O. Electron transport chain defects in heart failure.<br />
Heart Failure Rev. 2002;7:131-139.<br />
39. Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-<br />
ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy.<br />
Circulation. 1997;96:2190-2196.<br />
40. Neubauer S, Krahe T, Schindler R, et al. 31P magnetic resonance spectroscopy<br />
in dilated cardiomyopathy and coronary artery disease: altered cardiac<br />
high-energy phosphate metabolism in heart failure. Circulation. 1992;86:<br />
1810-1818.<br />
41. Kuo TH, Giacomelli F, Wiener J. Oxidative metabolism of Polytron versus<br />
Nagarse mitochondria in hearts of genetically diabetic mice. Biochim Biophys<br />
Acta. 1985;806:9-15.<br />
42. Kuo TH, Moore KH, Giacomelli F, Wiener J. Defective oxidative metabolism<br />
of heart mitochondria from genetically diabetic mice. Diabetes.<br />
1983;32:781-787.<br />
43. Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced<br />
mitochondrial oxidative capacity and increased mitochondrial uncoupling<br />
impair myocardial energetics in obesity. Circulation. 2005;112:<br />
2686-2695.<br />
44. Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant<br />
heart exhibits a mitochondrial biogenic response driven by the peroxisome<br />
proliferator-activated receptor- a /PGC-1 a gene regulatory pathway. Circulation.<br />
2007;115:909-917.<br />
45. Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced<br />
cardiac efficiency in diabetes. Physiology (Bethesda). 2006;21:250-<br />
258.<br />
46. Dong F, Zhang X, Yang X, et al. Impaired cardiac contractile function in<br />
ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol.<br />
2006;188:25-36.<br />
47. Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism.<br />
Trends Cardiovasc Med. 2000;10:238-245.<br />
48. Morrow D A, Givertz MM. Modulation of myocardial energetics: emerging<br />
evidence for a therapeutic target in cardiovascular disease. Circulation. 2005;<br />
112:3218-3221.<br />
49. Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the<br />
heart in obesity related diabetes: direct evidence for increased uncoupled respiration<br />
and activation of uncoupling proteins. Diabetes. 2007;56:2457-<br />
2466.<br />
50. Heaton GM, Wagenvoord RJ, Kemp Jr A, Nicholls DG. Brown-adiposetissue<br />
mitochondria: photoaffinity labelling of the regulatory site of energy dissipation.<br />
Eur J Biochem. 1978;82:515-521.<br />
51. Nicholls DG. Hamster brown-adipose-tissue mitochondria. Purine nucleotide<br />
control of the ion conductance of the inner membrane, the nature of<br />
the nucleotide binding site. Eur J.Biochem. 1976;62:223-228.<br />
52. Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol.<br />
Rev. 1984;64:1-64.<br />
53. Ledesma A, de Lacoba MG, Rial E. The mitochondrial uncoupling proteins.<br />
Genome Biol. 2002;3:REVIEWS3015.<br />
54. Fleury C, Neverova M, Collins S, et al. Uncoupling protein-2: a novel gene<br />
linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269-272.<br />
55. Gimeno RE, Dembski M, Weng X, et al. Cloning and characterization of<br />
an uncoupling protein homolog: a potential molecular mediator of human thermogenesis.<br />
Diabetes. 1997;46:900-906.<br />
56. Clapham JC, Arch JR, Chapman H, et al. Mice overexpressing human uncoupling<br />
protein-3 in skeletal muscle are hyperphagic and lean. Nature.<br />
2000;406:415-418.<br />
57. Vidal-Puig AJ, Grujic D, Zhang CY, et al. Energy metabolism in uncoupling<br />
protein 3 gene knockout mice. J Biol Chem. 2000;275:16258-16266.<br />
58. Echtay KS, Esteves TC, Pakay JL, et al. A signalling role for 4-hydroxy-<br />
2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:<br />
4103-4110.<br />
59. Echtay KS, Roussel D, St-Pierre J, et al. Superoxide activates mitochondrial<br />
uncoupling proteins. Nature. 2002;415:96-99.<br />
60. Murray AJ, Panagia M, Hauton D, Gibbons GF, Clarke K. Plasma free fatty<br />
acids and peroxisome proliferator-activated receptor a in the control of myocardial<br />
uncoupling protein levels. Diabetes. 2005;54:3496-3502.<br />
61. Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling<br />
proteins in human heart. Lancet. 2004;364:1786-1788.<br />
62. Acin A, Rodriguez M, Rique H, Canet E, Boutin JA, Galizzi JP. Cloning and<br />
characterization of the 5# flanking region of the human uncoupling protein 3<br />
(UCP3) gene. Biochem Biophys Res Commun. 1999;258:278-283.<br />
63. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated<br />
fatty acids, and eicosanoids are ligands for peroxisome proliferatoractivated receptors<br />
alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312-4317.<br />
Advances in Pulmonary Hypertension 335
64. Gilde AJ, van der Lee KA, Willemsen PH, et al. Peroxisome proliferatoractivated<br />
receptor (PPAR) alpha and PPARbetaq/delta, but not PPARgamma,<br />
modulate the expression of genes involved in cardiac lipid metabolism. Circ<br />
Res. 2003;92:518-524.<br />
65. Tu N, Chen H, Winnikes U, et al. Molecular cloning and functional characterization<br />
of the promoter region of the human uncoupling protein-2 gene.<br />
I. 1999;265:326-334.<br />
66. Roussel D, Chainier F, Rouanet J, Barre H. Increase in the adenine nucleotide<br />
translocase content of duckling subsarcolemmal mitochondria during<br />
cold acclimation. I. 2000;477:141-144.<br />
67. Skulachev VP. Anion carriers in fatty acid-mediated physiological uncoupling.<br />
J Bioenerg Biomembr. 1999;31:431-445.<br />
68. Nishikawa T, Edelstein D, Du XL, et al. <strong>No</strong>rmalizing mitochondrial superoxide<br />
production blocks three pathways of hyperglycaemic damage. Nature.<br />
2000;404:787-790.<br />
69. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M.<br />
Leptin induces mitochondrial superoxide production and monocyte chemoattractant<br />
protein-1 expression in aortic endothelial cells by increasing fatty acid<br />
oxidation via protein kinase A. J Biol Chem. 2001;276:25096-25100.<br />
70. Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin<br />
Clin Nutr Metab Care. 2002;5:561-568.<br />
71. Evans J L, Goldfine I D, Maddux BA, Grodsky GM. Are oxidative stress-activated<br />
signaling pathways mediators of insulin resistance and b-cell dysfunction?<br />
Diabetes. 2003;52:1-8.<br />
72. Rosen P, Du X, Sui GZ. Molecular mechanisms of endothelial dysfunction<br />
in the diabetic heart. Adv Exp Med Biol. 2001;498:75-86.<br />
73. Marra G, Cotroneo P, Pitocco D, et al. Early increase of oxidative stress and<br />
reduced antioxidant defenses in patients with uncomplicated type 1 diabetes:<br />
a case for gender difference. Diabetes Care. 2002;25:370-375.<br />
74. Van Dam PS, Van Asbeck, BS, Erkelens DW, Marx JJ, Gispen WH, Bravenboer<br />
B. The role of oxidative stress in neuropathy and other diabetic complications.<br />
Diabetes Metab Rev. 1995;11:181-192.<br />
75. Kristal BS, Jackson CT, Chung HY, Matsuda M, Nguyen HD, Yu,BP. Defects<br />
at center P underlie diabetes-associated mitochondrial dysfunction. Free<br />
Radical Biol Med. 1997;22:823-833.<br />
76. Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants<br />
prevent hyperglycemia-induced formation of intracellular advanced glycation<br />
endproducts in bovine endothelial cells. J Clin Invest. 1996;97:1422-1428.<br />
77. Lashin OM, Szweda PA, Szweda LI, Romani AM. Decreased complex II respiration<br />
and HNE-modified SDH subunit in diabetic heart. Free Radical Biol<br />
Med. 2006;40:886-896.<br />
78. Ye G, Metreveli NS, Donthi RV, et al. Catalase protects cardiomyocyte<br />
function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336-<br />
1343.<br />
79. Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria<br />
by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes.<br />
2006;55:798-805.<br />
80. Shen X, Zheng S, Thongboonkerd V, et al. Cardiac mitochondrial damage<br />
and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol<br />
Metab. 2004;287:E896-E905.<br />
81. Santos DL, Palmeira CM, Seica R, et al. Diabetes and mitochondrial oxidative<br />
stress: a study using heart mitochondria from the diabetic Goto-Kakizaki<br />
rat. Mol Cell Biochem. 2003;246:163-170.<br />
82. Conti M, Renaud IM, Poirier B, et al. High levels of myocardial antioxidant<br />
defense in aging nondiabetic normotensive Zucker obese rats. Am J Physiol<br />
Regul Integr Comp Physiol. 2004;286:R793-R800.<br />
83. Vincent HK, Powers SK, Stewart DJ, Shanely RA, Demirel H, Naito H.<br />
Obesity is associated with increased myocardial oxidative stress. Int J Obes<br />
Relat Metab Disord. 1999;23:67-74.<br />
84. Bugger H, Boudina S, Hu XX, et al. Type 1 diabetic akita mouse hearts<br />
are insulin sensitive but manifest structurally abnormal mitochondria that remain<br />
coupled despite increased uncoupling protein 3. Diabetes. 2008;57:<br />
2924-232.<br />
85. Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic<br />
calcium. J Mol Cell Cardiol. 2002;34:1259-1271.<br />
86. Bouchard RA, Bose D. Influence of experimental diabetes on sarcoplasmic<br />
reticulum function in rat ventricular muscle. Am J Physiol. 1991;260:<br />
H341-H354.<br />
87. Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D. Altered Ca2+<br />
handling in ventricular myocytes isolated from diabetic rats. Am J Physiol.<br />
1996;270:H1529-H1537.<br />
88. Penpargkul S, Fein F, Sonnenblick EH, Scheuer J. Depressed cardiac sarcoplasmic<br />
reticular function from diabetic rats. J Mol Cell Cardiol. 1981;13:<br />
303-309.<br />
89. Netticadan T, Temsah RM, Kent A, Elimban V, Dhalla NS. Depressed levels<br />
of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction<br />
in the diabetic heart. Diabetes. 2001;50:2133-2138.<br />
90. Russ M, Reinauer H, Eckel J. Diabetes-induced decrease in the mRNA<br />
coding for sarcoplasmic reticulum Ca2+-ATPase in adult rat cardiomyocytes.<br />
Biochem Biophys Res Commun. 1991;178:906-912.<br />
91. Zhong Y, Ahmed S, Grupp IL, Matlib MA. Altered SR protein expression<br />
associated with contractile dysfunction in diabetic rat hearts. Am J Physiol.<br />
2001;281:H1137-H1147.<br />
92. Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression<br />
of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial<br />
contractility in diabetic cardiomyopathy. Diabetes. 2002;51:1166-1171.<br />
93. Vetter R, Rehfeld U, Reissfelder C, et al. Transgenic overexpression of the<br />
sarcoplasmic reticulum Ca2+ATPase improves reticular Ca2+ handling in normal<br />
and diabetic rat hearts. FASEB J. 2002;16:1657-1659.<br />
94. Belke DD, Betuing S, Tuttle MJ, et al. Insulin signaling coordinately regulates<br />
cardiac size, metabolism, and contractile protein isoform expression. J<br />
Clin Invest. 2002;109:629-639.<br />
95. Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally<br />
invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling<br />
during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:<br />
H1261-H1269.<br />
96. McQueen AP, Zhang D, Hu P, et al. Contractile dysfunction in hypertrophied<br />
hearts with deficient insulin receptor signaling: possible role of reduced<br />
capillary density. J Mol Cell Cardiol. 2005;39:882-892.<br />
336 Advances in Pulmonary Hypertension
Continuing Medical Education Section<br />
Specialized Centers of Clinically<br />
Oriented Research Programs in<br />
Pulmonary Hypertension Reported<br />
Progress at the <strong>PHA</strong> Scientific Sessions<br />
Karen A. Fagan, MD, Guest Editor<br />
<strong>University</strong> of South Alabama College of Medicine<br />
Mobile, Alabama<br />
Recently, the National Heart, Lung, and Blood Institute<br />
awarded 2 Specialized Centers of Clinically Oriented Research<br />
(SCCOR) program grants in pulmonary hypertension.<br />
The SCCOR program requires clinical and basic scientists<br />
with a broad range of skills to work together on a unified<br />
theme, with special emphasis on clinically relevant research.<br />
The goal of the SCCOR program is to encourage multidisciplinary<br />
research on clinically relevant problems to allow<br />
basic science findings to be more rapidly applied to clinical<br />
situations. It is expected that over 50% of the funded research<br />
is clinical and interactions between clinical and basic<br />
scientists are expected to strengthen the research, enhance<br />
the translation of fundamental research findings to the clinical<br />
setting, and identify new research directions. In addition,<br />
each SCCOR project must have a defined organizational and<br />
administrative structure to enhance and enable interactions<br />
between investigators to increase the rate of translation of<br />
basic research findings to clinical applications.<br />
At the recent Scientific Sessions at the Pulmonary Hypertension<br />
Association 8 th International Conference, the principal<br />
investigators for the 2 SCCOR programs in pulmonary<br />
hypertension—Dr Paul Hassoun from Johns Hopkins <strong>University</strong><br />
and Dr Kurt Stenmark from the <strong>University</strong> of Colorado<br />
Denver School of Medicine, reported on progress made in<br />
each of their research programs. These are summarized in<br />
the following reports.<br />
National Heart Lung and Blood Institute<br />
Hopkins Specialized Center in Clinical Oriented<br />
Research (SCCOR): Molecular Determinants of<br />
Pulmonary Arterial Hypertension<br />
Paul M. Hassoun, MD<br />
Professor of Medicine and<br />
Director of the Pulmonary Hypertension Program<br />
Division of Pulmonary and Critical Care Medicine<br />
Johns Hopkins <strong>University</strong>, School of Medicine<br />
Baltimore, MD<br />
Paul M. Hassoun, MD<br />
SCCOR Investigators: Paul M. Hassoun: overall Principal Investigator,<br />
Project 1 leader and administrative core leader; Hunter C.<br />
Champion: Project 2 leader; Fredrick Wigley: Project 3 leader;<br />
Roger A. Johns: Project 4 leader; Michael Crow: Project 5 leader;<br />
<strong>No</strong>ah Lechtzin: data management and statistics; Allen Myers:<br />
pathology core; Kathleen C. Barnes: genetics/genomics core; Jennifer<br />
van Eyk: proteomics core; and Jens Vogel-Claussen: imaging<br />
core.<br />
Pulmonary arterial hypertension (PAH) is the leading cause of mortality<br />
in patients with the spectrum of scleroderma-related diseases.<br />
In addition, recent large clinical trials of PAH suggest that<br />
patients with scleroderma-related PAH have increased mortality<br />
and a significantly poorer response to therapy compared with patients<br />
who have idiopathic PAH. Although the reason for this discrepancy<br />
remains unclear, we hypothesized for this SCCOR that<br />
the overall worse outcome in scleroderma-related PAH is related<br />
to more severe structural changes involving the pulmonary vasculature<br />
(PV) and the right ventricle (RV), resulting in marked RV-<br />
PV dysfunction. Therefore, this SCCOR project is focused on<br />
understanding the complex PV and RV remodeling, resulting RV-<br />
PV uncoupling, and their crucial impact on morbidity and mortality<br />
in PAH.<br />
In this SCCOR, we use scleroderma-related PAH as a clinical<br />
paradigm, contrasting it to idiopathic PAH, because of its particular<br />
severity, lack of response to available PAH therapy, and potential<br />
underlying genetic factors that dictate outcome. Because<br />
of the extensive expertise of our team in molecular and diagnostic<br />
pulmonary medicine and cardiology, we have the unique opportunity<br />
to not only characterize RV-PV responses in scleroderma-related<br />
PAH with increased sensitivity and clarity, but to<br />
also identify new molecular targets for potential therapy using<br />
state of the art imaging and genomic and proteomic technology.<br />
Relying on novel imaging systems and molecular tools, we proposed<br />
to conduct rigorous phenotypic characterization of patients<br />
who have scleroderma-related PAH. Our focus on animal models<br />
provides us with additional candidate genes and proteins for characterization<br />
and targeting in human studies.<br />
We have the opportunity to validate the clinical importance of<br />
these genes in a large cohort of well-phenotyped patients with<br />
PAH, using functional genomics and proteomic approaches with<br />
characterization of potentially important polymorphisms. We hope<br />
Advances in Pulmonary Hypertension 337
Survival probablility (%)<br />
13<br />
12<br />
11<br />
10<br />
9<br />
27<br />
26 25<br />
8<br />
7<br />
6 5<br />
4<br />
5<br />
3<br />
24<br />
5/27 patients<br />
25<br />
23<br />
2<br />
22<br />
0<br />
0<br />
11/13 patients<br />
Delayed Enhancement<br />
Mass (g/m 2 )<br />
Figure 2. Gadolinium delayed enhancement is seen essentially at RV insertion<br />
site. Right graph shows no difference in scar mass between idiopathic<br />
PAH and PAH-SS patients.<br />
<strong>No</strong>te: 11 of 13 patients with hyponatremia who died had sclerodermarelated<br />
PAH.<br />
4.0<br />
3.0<br />
2.0<br />
1.0<br />
0<br />
IPAH<br />
PAH-SSc<br />
Time (months)<br />
PAH-SSc<br />
Figure 1. Kaplan and Meier estimates of survival (all-cause mortality)<br />
in patients stratified by serum sodium.<br />
that our data will provide new insights into the molecular basis for<br />
rational strategies for patients who have scleroderma-related PAH,<br />
and elucidate the relationship of RV-PV dysfunction to the activation<br />
of pathological gene expression in genetically susceptible<br />
patients.<br />
In summary, the Hopkins SCCOR application represents a consortium<br />
of investigators with multidisciplinary expertise. The common<br />
goal to use state-of-the-art physiological, molecular, and<br />
genomic and proteomic approaches as well as novel phenotyping<br />
instrumentation that will provide the deepest understanding of<br />
the critical pathobiological processes of RV-PV dysfunction and<br />
uncoupling to date, and define key genetic determinants relevant<br />
to scleroderma-related PAH. The 5 human and animal projects<br />
are supported by 6 highly interactive cores (administration, data<br />
management/bioinformatics, molecular pathology, genomic and<br />
genotyping, proteomics, and imaging). We anticipate our work will<br />
provide a foundation for meaningful translational research that<br />
will facilitate development of new strategies, uncover therapeutic<br />
targets, and define new biomarkers and prognostic indicators that<br />
will limit the current dismal outcome of scleroderma-associated<br />
PAH.<br />
Progress<br />
The major goals of this SCCOR project are to develop reliable<br />
measures of RV-PV function, to characterize patterns of gene expression<br />
and identify candidate gene polymorphisms associated<br />
with susceptibility to PAH, and to use these tools to guide therapy<br />
aimed at RV-PV dysfunction in scleroderma-related PAH. As part<br />
of our SCCOR activities, we have recently demonstrated that hyponatremia<br />
is a significant indicator of survival (Figure 1) in patients<br />
with PAH, in particular in patients with scleroderma-related<br />
PAH. 1 Hyponatremia is 9 times more likely to be present in scleroderma-related<br />
PAH when controlling for hemodynamics and renal<br />
function, which suggests that up-regulation of the renine-aldosterone-angiotensin<br />
system (RAAS) in response to hemodynamic<br />
stress from PAH differs between idiopathic PAH and sclerodermarelated<br />
PAH. Based on this and other clinical findings that indicate<br />
the involvement of RAAS activation in scleroderma-related PAH,<br />
some members of our team are focusing their effort on genes pertinent<br />
to neurohormonal activation such as adreno-medullin.<br />
Characterizing RV-PV Function<br />
To characterize optimal measures of RV-PV function in scleroderma-related<br />
PAH we use a combination of hemodynamic data<br />
obtained from right heart catheterization data, echocardiographic<br />
parameters, and cardiac MRI with gadolinium imaging and stress<br />
test (adenosine infusion). We compare these data to patients with<br />
idiopathic PAH. RV function is an important determinant of prognosis<br />
in pulmonary hypertension as it is the single most significant<br />
prognostic marker of survival. In a prospectively studied cohort of<br />
63 consecutive patients with PH who were referred for a clinically<br />
indicated right heart catheterization we demonstrated that the degree<br />
of tricuspid annular displacement (tricuspid annular plane<br />
systolic excursion or TAPSE) powerfully reflects RV function and<br />
prognosis in PAH. 2 Specifically, we demonstrated that a low<br />
TAPSE value of less than 1.8 cm was associated with greater RV<br />
systolic dysfunction, more RV remodeling, and right ventricle-left<br />
ventricle disproportion. More importantly, this study demonstrated<br />
that TAPSE could predict survival when these patients were followed<br />
over time on therapy. This is now a widely quoted study<br />
among the PH community.<br />
In addition, we have focused on several cardiac MRI parameters<br />
obtained prospectively and within 2 to 4 hours of right heart<br />
catheterization. Pulmonary distensibility is of interest because of<br />
the potential of increased fibrosis that can cause stiffening of the<br />
proximal pulmonary arteries in scleroderma-related PAH and contribute<br />
to RV-PV uncoupling. This analysis has generated some<br />
intriguing results comparing scleroderma patients with and without<br />
PAH and controls. We have also focused on myocardial scarring<br />
in PAH patients and postulated that patients who have<br />
scleroderma-related PAH might have increased scar mass compared<br />
to patients with idiopathic PAH. Although we found no difference<br />
in scar mass between the 2 groups (Figure 2), scar mass<br />
as assessed by cardiac MRI correlated strongly with RV end diastolic<br />
volume in patients with scleroderma-related PAH but not in<br />
those with idiopathic PAH (Table).<br />
Candidate Genes for Scleroderma-Related PAH<br />
We have published our first observation for the use of genomic<br />
profiling in patients with scleroderma-related PAH (compared with<br />
patients who have idiopathic PAH). Briefly, we hypothesized that<br />
PAH-associated genes identified by expression profiling of peripheral<br />
blood mononuclear cells from patients with idiopathic<br />
PAH can also be identified in peripheral blood mononuclear cells<br />
338 Advances in Pulmonary Hypertension
Table. Correlation of Hemodynamic and MRI<br />
Morphology Variables With Scar Mass in<br />
Scleroderma-Related PAH<br />
r<br />
P<br />
Right ventricular –0.391 NS<br />
ejection fraction<br />
Right ventricular end 0.729 NS<br />
diastolic mass index<br />
Right ventricular end 0.970 .0014<br />
diastolic volume index<br />
Mean pulmonary 0.600 NS<br />
artery pressure<br />
Pulmonary vascular 0.682 NS<br />
resistance<br />
Cardiac index –0.294 NS<br />
Figure 3. Color display of genes discriminating between idiopathic PAH and<br />
PAH-SS versus controls and assorted according to disease severity. Red, increased<br />
expression versus control; green, lower expression.<br />
from scleroderma-related PAH. Gene expression profiles of peripheral<br />
blood mononuclear cells collected from patients with idiopathic<br />
PAH, those with scleroderma-related PAH, and healthy<br />
controls were generated using HG_U133A_2.0 GeneChips. Disease<br />
severity in consecutive patients was assessed by functional<br />
status and hemodynamic measurements. As shown in Figure 3,<br />
there were many genes that were up- or down-regulated concordantly<br />
or not in the 2 groups. Our data demonstrate that peripheral<br />
blood mononuclear cells from patients with sclerodermarelated<br />
PAH carry distinct transcriptional expression. 3 Deciphering<br />
the role of genes involved in vascular remodeling and PAH de-
velopment may reveal novel targets for treatment for this devastating<br />
disorder, which is one of the most important goals of this<br />
SCCOR project.<br />
Biological Validation<br />
To establish biological validation of high-risk alleles in selected<br />
PAH candidate genes via mid- and high-throughput genotyping in<br />
a large cohort of scleroderma-related PAH patients we have collected<br />
over 1400 DNA samples (mostly patients with scleroderma<br />
of whom 10% have scleroderma-related PAH). We used these<br />
samples for further DNA analysis and to identify single nucleotide<br />
polymorphisms of candidate genes identified in our SCCOR projects<br />
dedicated to human and animal studies. We have also established<br />
collaborations with other investigators from the PH<br />
community to share additional DNA samples. We will begin high<br />
throughput genotyping as early as December 1, 2008. The goal is<br />
to perform wide-scale single nucleotide polymorphisms analysis<br />
on several candidate genes (from a current total list of<br />
39 genes).<br />
Conclusion<br />
We continue to gain significant momentum and synergy with other<br />
SCCOR investigators and have moved all aspects of our project<br />
forward. We anticipate that the coming year will be, like this past<br />
year, extremely productive now that we have most of our techniques<br />
and analytical tools in place. More importantly, we hope<br />
that our efforts will help clarify the pathobiology underlying scleroderma-related<br />
PAH and its current poor outcome and identify<br />
new molecular targets for the design of targeted therapies.<br />
References<br />
1. Forfia P, Mathai SC, Fisher MR, et al. Hyponatremia predicts right heart failure<br />
and poor survival in pulmonary arterial hypertension. Am J Respir Crit<br />
Care Med. 2008;177:1364-1369.<br />
2. Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement<br />
predicts survival in pulmonary hypertension. Am J Respir Crit Care Med.<br />
2006;174:1034-41.<br />
3. Grigoryev DN, Fisher MR, Mathai SC, et al. Identification of candidate genes<br />
in scleroderma-related pulmonary arterial hypertension. Transl Res. 2008;151:<br />
197-207.<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
340 Advances in Pulmonary Hypertension
Continuing Medical Education Section<br />
Specialized Center in Clinical Oriented Research<br />
(SCCOR) Update: Mechanisms and Treatment of Lung<br />
Vascular Disease in Infants and Children<br />
Kurt Stenmark, MD<br />
Department of Pediatrics<br />
Developmental Lung Biology Laboratory<br />
<strong>University</strong> of Colorado at Denver and Health Sciences Center<br />
Aurora, Colorado<br />
Kurt Stenmark, MD<br />
SCCOR Investigators: John Kinsella: Principal Investigator (PI)<br />
Project 1; Robin Shandas, PI, Dunbar Ivy, Co-PI: Project 2; Kurt<br />
Stenmark. PI: Project 3; Carl White, PI: Project 4.<br />
In contrast to lung branching morphogenesis, studies of the<br />
mechanisms that regulate lung vascular development and that<br />
link capillary growth with alveolarization are relatively recent and<br />
limited in scope. Lack of information regarding lung vascular<br />
growth and its connection with alveolar growth is unfortunate,<br />
because developmental abnormalities of the pulmonary circulation<br />
contribute to the pathogenesis of several important neonatal<br />
cardiopulmonary disorders including pulmonary hypertension<br />
(PH) in the newborn.<br />
There is growing recognition that the importance of understanding<br />
basic mechanisms of lung vascular growth in the context<br />
of human disease may be best highlighted in the setting of<br />
bronchopulmonary dysplasia (BPD). BPD is a significant health<br />
care problem associated with acute and long-term pulmonary<br />
consequences.<br />
Recent data from animal and clinical studies suggest that impaired<br />
vascular growth may contribute to abnormalities of lung architecture,<br />
especially decreased alveolarization, and thus play a<br />
critical role in the pathogenesis of BPD. However, little is known<br />
about the mechanisms of pulmonary vascular injury in the immature<br />
lung, the impact of this injury on growth and development<br />
of the lung, or its contribution to the pathogenesis of BPD<br />
and PH.<br />
The overall goal of this SCCOR project is to generate clinical<br />
and basic information that will provide insight into the mechanisms<br />
contributing to pulmonary vascular abnormalities that<br />
characterize BPD, to evaluate currently available therapies aimed<br />
at reducing lung injury and restoring vascular and lung growth,<br />
and to examine in animal models new approaches to ameliorate<br />
perinatal lung injury and restore vascular and lung growth. Two<br />
clinical and 2 basic projects address these objectives. The clinical<br />
projects evaluate the impact of inhaled nitric oxide (iNO) on<br />
BPD and the development of improved techniques to assess the<br />
presence of PH and the responses to therapy in infants with PH.<br />
The 2 basic projects dissect the mechanisms that contribute to<br />
lung vascular remodeling in murine, rodent, ovine, and bovine<br />
models and evaluate the effects of novel pharmacological agents<br />
on lung vascular disease in these models. The long-term goal is<br />
to use information derived from these models to develop new and<br />
improved therapies for the infant with BPD and/or PH.<br />
<strong>No</strong>ninvasive Inhaled NO in Premature Newborns<br />
Project 1 is a randomized, placebo-controlled and masked pilot<br />
trial of low-dose, noninvasive iNO in premature newborns (500-<br />
1250 grams birth weight) that do not require intubation for respiratory<br />
failure in the first 36 hours of life. The rationale and<br />
background for this study have been recently summarized. 1,2 The<br />
aims of the study are to determine if iNO reduces BPD/mortality<br />
in premature newborns who do not require intubation in the first<br />
24 hours of life and to determine if noninvasive iNO treatment<br />
decreases early and late pulmonary vascular abnormalities in this<br />
population.<br />
Advanced Imaging and Diagnostics for Pediatric PH<br />
The overall goal of Project 2 is to develop and evaluate more<br />
comprehensive measures of pulmonary arterial hypertension<br />
using a combination of advanced cardiovascular imaging and sophisticated<br />
computational modeling. The overall hypothesis for<br />
these studies is that pulmonary vascular input impedance provides<br />
a more comprehensive measure of pulmonary vascular<br />
function than pulmonary vascular resistance (PVR) alone since<br />
impedance includes both dynamic (stiffness or compliance) and<br />
steady state (resistance) components of the vascular circuit.<br />
Measurement of PVR is the current standard for evaluating<br />
PH and pulmonary vascular reactivity in children with pulmonary<br />
arterial hypertension (PAH). However, PVR measures only the<br />
mean component of right ventricular afterload and neglects pulsatile<br />
or dynamic effects. Increased stiffness in the pulmonary<br />
vasculature is increasingly appreciated to affect right ventricular<br />
afterload and to perpetuate distal pulmonary vascular disease.<br />
The investigators in this project, therefore, recently developed<br />
and validated a method to measure pulmonary vascular input impedance<br />
(a parameter which evaluates dynamic [stiffness] and resistive<br />
components of the vasculature) and demonstrated excel-<br />
Advances in Pulmonary Hypertension 341
lent correlation between impedance measurements and PVR as<br />
well as a correlation between impedance measurements and pulmonary<br />
vascular stiffness. 3,4<br />
The investigators have demonstrated that impedance can be<br />
measured routinely and easily in the cardiac catheterization laboratory.<br />
Most importantly, the investigators have demonstrated<br />
that impedance is a better predictor of disease outcome in pediatric<br />
patients with PAH than is simple measurement of PVR. 4<br />
Similar observations have been made in adult studies of PH by<br />
the SCCOR program at Johns Hopkins. They have demonstrated<br />
that impedance is a better and more effective way of evaluating<br />
PH than measuring PVR alone. Work in this project may establish<br />
improved methods to evaluate and follow the impact of pharmacological<br />
interventions in patients with PAH.<br />
Circulating Fibrocytes in Hyperoxic Lung<br />
Vascular Remodeling<br />
The long-term goal of Project 3 is to determine the role of circulating<br />
fibrocytes (precursors of mesenchymal cells) in neonatal<br />
lung vascular remodeling. There is good evidence that mesenchymal<br />
progenitor cells are recruited to the injured lung in<br />
young animals (mice, rats, calves) and play important roles in<br />
the pulmonary hypertensive process. 5 There are few data that<br />
demonstrate recruitment of progenitor cells to the vasculature of<br />
humans with PAH. Therefore, in collaboration with one of the<br />
major groups investigating adult PH (Vanderbilt <strong>University</strong>), tissues<br />
from patients with severe PAH were evaluated to determine<br />
the presence of cells expressing progenitor cell markers (CD133).<br />
A significant increase in the accumulation of CD133+ cells<br />
both in intimal lesions and in the perivascular regions of pulmonary<br />
arteries from patients with severe PH was observed. Because<br />
questions have arisen as to how these cells might affect<br />
vascular structure or function, we evaluated the possibility that<br />
they exerted their effects through a process of cell fusion and/or<br />
heterokaryon formation. This is one mechanism through which<br />
stem cells are often thought to exert their effects. Extensive<br />
analysis, however, did not demonstrate any evidence for fusion of<br />
these recruited cells to local vascular cells. 6 The recruited inflammatory/progenitor<br />
cells appear to exert effects on structure<br />
and function of blood vessels through processes other than cell<br />
fusion.<br />
These are important findings because they demonstrate that<br />
human PAH is associated with progenitor cell recruitment just<br />
as has been shown in animal models. We are currently collecting<br />
tissues from human infants with PH to carry out similar studies.<br />
In addition, we continue our efforts to determine the mechanisms<br />
through which inflammatory cells and progenitor cells are<br />
recruited to the lung. We are following up on our observations<br />
demonstrating that superoxide radical (O 2-<br />
) plays a critical role in<br />
initiating and perpetuating the remodeling process in the injured<br />
lung. 7,8 Having shown that transgenic overexpression of EC-SOD<br />
attenuated superoxide-induced signaling and dramatically attenuated<br />
PH and remodeling, we have embarked on studies (in<br />
collaboration with the <strong>University</strong> of Colorado Denver) to evaluate<br />
the effects of EC-SOD mimetics in rodent models of PH. 7<br />
Hypoxia-Inducible Factors in Neonatal PH<br />
The long-term goal of Project 4 is to develop agents that can<br />
specifically increase lung vascularization and thereby restore<br />
alveolarization to more normal levels. In important background<br />
studies the investigators established that hypoxia inducible factors<br />
(HIFs), important regulators of vascular endothelial growth<br />
factor (VEGF), are decreased in experimental acute lung injury. 9<br />
The investigators are testing the hypothesis that prolylhydroxylase<br />
inhibitors (PHDI), agents that stabilize the transcription factor<br />
HIF, can decrease PH in the newborn by restoring the fetal VEGF/<br />
eNOS axis. 10<br />
Unfortunately, in preliminary experiments, PHDIs were found<br />
to cause increased lethality in premature baboons, apparently due<br />
to immunomodulation with an exuberant inflammatory response<br />
(unpublished observation). These effects were thought to be due<br />
to overexpression of HIF1α. Therefore, the investigators have<br />
worked to develop methods to selectively activate HIF2α in the<br />
hopes that activation of this pathway will selectively result in protective<br />
angiogenic effects. The investigators have developed targeted<br />
stabilization of HIF2α, and they have demonstrated that<br />
overexpression of HIF2α increases adenosine A 2A<br />
receptor expression.<br />
Importantly, the investigators show that overexpression<br />
of adenosine A 2A<br />
receptor in endothelial cells can increase endothelial<br />
cell proliferation and endothelial branching. Thus, significant<br />
progress is being made in determining the mechanisms<br />
through which transcription factors can be selectively manipulated<br />
to achieve the desired beneficial effects in the neonatal lung.<br />
References<br />
1. Kinsella, JP. Inhaled nitric oxide in the term newborn. Early Hum Dev.<br />
2008;84:709-716.<br />
2. Kinsella JP, Abman SH. Inhaled nitric oxide in the premature newborn. J<br />
Pediatr. 2007;151:10-15.<br />
3. Hunter KS, Gross JK, Lanning CJ, et al. <strong>No</strong>ninvasive methods for determining<br />
pulmonary vascular function in children with pulmonary arterial hypertension:<br />
application of a mechanical oscillator model. Cong Heart Dis.<br />
2008;3:106-116.<br />
4. Hunter KS, Lee PF, Lanning CJ, et al. Pulmonary vascular input impedance<br />
is a combined measure of pulmonary vascular resistance and stiffness and<br />
predicts clinical outcomes better than PVR alone in pediatric patients with<br />
pulmonary hypertension. Am Heart J. 2008;155:166-174.<br />
5. Frid MG, Brunetti JA, Burke DL, et al. Hypoxia-induced pulmonary vascular<br />
remodeling requires recruitment of circulating mesenchymal precursors of a<br />
monocyte/macrophage lineage. Am. J. Pathol. 2006;168:659-669.<br />
6. Majka SM, Skokan M, Wheeler L, et al. Evidence for cell fusion is absent<br />
in vascular lesions associated with pulmonary arterial hypertension. Am J<br />
Physiol Lung Cell Mol Physiol. 2008;295:L1028-L1039. Epub 2008 Oct 17.<br />
7. <strong>No</strong>zik-Grayck E, Suliman HB, Majkaa SM, et al. Lung EC-SOD overexpression<br />
attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary<br />
vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:<br />
L422-L430.<br />
8. <strong>No</strong>zik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic<br />
hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp<br />
Med Biol. 2007;618:101-112.<br />
9. Grover TR, Asikainen TM, Kinsella JP, Abman SH, White CW. Hypoxia-inducible<br />
factors HIF-1alpha and HIF-2alpha are decreased in an experimental<br />
model of severe respiratory distress syndrome in preterm lambs. Am J<br />
Physiol Lung Cell Mol Physiol. 2007;292:L1345-51. Epub 2007 Feb 16.<br />
10. Asikainen TM, White CW. HIF stabilizing agents: shotgun or scalpel? Am<br />
J Physiol Lung Cel. Mol Physiol. 2007;293:L555-L556.<br />
342 Advances in Pulmonary Hypertension
Continuing Medical Education Section<br />
Getting More From Right Heart Catheterization:<br />
A Focus on the Right Ventricle<br />
Hunter C. Champion, MD, PhD<br />
Pulmonary Hypertension Program<br />
and Division of Cardiology<br />
Department of Medicine<br />
Johns Hopkins Medical Institutions<br />
Baltimore, MD<br />
Hunter C.<br />
Champion, MD, PhD<br />
The primary challenge in the care of the patient with advanced<br />
pulmonary hypertension (PH) is right ventricular dysfunction with<br />
concomitant right heart failure, which is the most important cause<br />
of mortality in the disease. It is increasingly evident that the interaction<br />
of the heart and pulmonary circulation is a very important<br />
aspect that is largely understudied and our previous<br />
assessment of right ventricular function has been relatively crude.<br />
Here, we highlight the future of integrative assessment of ventricular-pulmonary<br />
vascular coupling via hemodynamic measures.<br />
Current Use of Right Heart Catheterization<br />
Cardiac catheterization remains the gold standard for diagnosing<br />
pulmonary hypertension, assessing disease severity, and determining<br />
prognosis and response to therapy. By directly measuring<br />
pressures and indirectly measuring flow, right heart catheterization<br />
allows for determination of prognostic markers such as right<br />
atrial pressure, cardiac output, and mean pulmonary artery pressure.<br />
1 This procedure has been shown to be safe, with no deaths<br />
reported in the NIH registry study. 1 In addition, a recent study reported<br />
a procedure-related mortality of 0.055%. 2<br />
Right heart catheterization determines the presence or absence<br />
of pulmonary hypertension, may define the underlying etiology,<br />
and allows for prognostication. The most critical aspect of<br />
right heart catheterization is that it is performed appropriately,<br />
and the data are interpreted accurately. Since end-expiratory intrathoracic<br />
pressure most closely correlates with atmospheric pressure,<br />
it is important that all right ventricular, pulmonary artery,<br />
pulmonary wedge, and left ventricular pressures be measured at<br />
end-expiration. 3-5 This is especially true in patients in whom there<br />
can be significant variation between inspiratory and end-expiratory<br />
vascular pressures (obese patients and patients with intrinsic lung<br />
disease).<br />
After determination of the presence of PH, pulmonary venous<br />
pressures should be evaluated by the pulmonary capillary wedge<br />
Address for reprints and other correspondence: Hunter C. Champion, MD,<br />
PhD, FAHA, FPVRI, Assistant Professor of Medicine, Division of Cardiology,<br />
Department of Medicine, Johns Hopkins Medical Institutions, 720 Rutland<br />
Avenue, Ross 835, Baltimore, MD 21205-2109; email: hcc@jhmi.edu.<br />
pressure (PCWP). Pulmonary arterial hypertension (PAH) is defined<br />
by a PCWP of 15 mmHg or less. 5,6 This value is based on the<br />
normal PCWP or left ventricular end diastolic pressure (LVEDP) of<br />
less than 8 mmHg and the observation that ~14 mmHg is 2 standard<br />
deviations from a normal PCWP. 3<br />
With the exception of patients with severe tricuspid regurgitation,<br />
both thermodilution and Fick methods are reliable in patients<br />
with PAH for the measurement of cardiac output. 7<br />
Vasodilator challenges with inhaled nitric oxide or intravenous<br />
epoprostenol or adenosine are encouraged in all patients at the<br />
time of diagnosis and in follow-up studies. 3<br />
Other Testing During Right Heart Catheterization<br />
Exercise and fluid challenge<br />
Some patients with pulmonary vascular disease are not symptomatic<br />
at rest, but have symptoms with exertion. This observation<br />
provides a potential for exercise or volume challenge during right<br />
heart catheterization to better diagnose early pulmonary vascular<br />
disease. In patients with risk factors for nonsystolic left ventricle<br />
(LV) dysfunction (sleep disordered breathing, systemic hypertension,<br />
obesity, diabetes/glucose intolerance) one should consider<br />
confrontational testing (to uncover potential increases in PCWP)<br />
by administering a fluid bolus challenge or exercise during right<br />
heart catheterization particularly if the patient has a resting PCWP<br />
between 8 and 15 mmHg.<br />
With regard to the threshold of a mean pulmonary arterial pressure<br />
(PAP) of 30 mmHg with exercise, the data to support this as<br />
a disease state that is similar to resting PAH are much less robust.<br />
The number of pulmonary hemodynamic studies that include<br />
exercise are made up of a smaller number of patients. 8<br />
Exercise pulmonary hemodynamics have been reported in 218<br />
healthy subjects (125 in one study of subjects aged 14 to 69<br />
years). 8-10<br />
The purpose of exercise is not only to examine pulmonary arterial<br />
pressure in response to exertion. Rather, the benefit of confrontational<br />
testing is the observation of the change/increase in<br />
PCWP in an effort to diagnose pulmonary venous hypertension or<br />
nonsystolic heart failure. Although protocols for exercise and work-<br />
Advances in Pulmonary Hypertension 343
a<br />
Figure. (a) Schematic showing the measurement of augmentation index using the pulmonary arterial waveform.<br />
This augmentation index (∆P/PAPP) relates the change in pressure (∆P) to the pulmonary arterial pulse pressure<br />
(PAPP) and gives an estimation of pulmonary vascular stiffness. (b) Schematic showing a sample RV pressure<br />
volume loop relationship including effective arterial elastance (Ea), end-systolic pressure volume relation (ESPVR),<br />
and end diastolic pressure volume loop relationship (EDPVR).<br />
load vary from study to study, and maximal workload exercise has<br />
been tested in few subjects, the main goal of exercise is to increase<br />
heart rate to 85% maximal age-predicted heart rate as is<br />
used in cardiology stress testing. Given increased thoracic pressure<br />
changes with exercise, particularly in overweight and/or deconditioned<br />
patients, it is critical that measurements be made at<br />
end-expiration to ensure uniformity in interpretation.<br />
An increase in PCWP to greater than 15 mmHg in response to<br />
exercise or fluid challenge suggests the presence of pulmonary<br />
venous hypertension, a condition with dramatically different management<br />
than PAH. Because cardiac output can increase up to 5-<br />
fold above baseline, pulmonary vascular resistance (PVR) normally<br />
decreases with exercise. 8,9 Poor prognostic signs in exercise right<br />
heart catheterization are: (1) the inability of the right ventricle<br />
(RV) to augment in response to exercise, ie, lack of a significant<br />
increase in cardiac output; (2) angina; and (3) presyncopal symptoms<br />
or frank syncope.<br />
<strong>No</strong>vel Hemodynamic Techniques<br />
Assessment of the pulmonary arterial pressure waveform. Chronic<br />
pulmonary hypertension results from an increase in pulmonary<br />
vascular resistance, which is a simple measure of the opposition<br />
to the mean component of flow. However, given the low resistance/high<br />
compliance nature of the pulmonary circulation, the<br />
pulsatile component of hydraulic load is also critical to consider.<br />
The fact that the mean and the pulsatile components of flow are<br />
dependent on different portions of the pulmonary circulation suggests<br />
that they can be controlled separately, without much overlap.<br />
The pulmonary circulation is pulsatile with multiple bifurcations;<br />
and wave reflection is an inevitable consequence. When the<br />
forward pressure wave from the heart collides with the backward<br />
pressure wave that was reflected from the bifurcations, pressure<br />
increases and flow decreases. Because the often used PVR only<br />
takes into account mean flow, it does not allow for changes in<br />
pulsatility of the pulmonary circuit. 11-14 One must consider the<br />
elastic properties of the pulmonary circulation and impedance on<br />
RV performance rather than the pure resistive properties since the<br />
heart could not function if it were not for the elastic properties of<br />
b<br />
pulmonary vasculature. During<br />
systole, the pulmonic valve is<br />
open at a time when the mitral<br />
valve is closed. Thus, if it were<br />
not for the elastic properties of<br />
the pulmonary vasculature, the<br />
heart could not develop forward<br />
flow. 12-14<br />
Pulse pressure indicates the<br />
amplitude of pulsatile stress.<br />
Pulse pressure is mainly determined<br />
by both the characteristics<br />
of ventricular ejection and arterial<br />
compliance, so that the lower<br />
the compliance, the higher the<br />
pulse pressure. Moreover, pressure<br />
waveform analysis performed<br />
in the time-domain makes<br />
it possible to calculate the timing<br />
and extent of wave reflection<br />
in systemic and pulmonary circulation<br />
using measures such as<br />
augmentation index (as shown in the Figure) which roughly represents<br />
reflected wave summation (∆P) in the pulmonary circuit<br />
and normalizes for pulmonary arterial pulse pressure. 15-23<br />
These values can be easily obtained at the time of right heart<br />
catheterization and the future studies will compare both analyses<br />
as potential prognostic indicators in patients with pulmonary hypertension.<br />
24<br />
Right ventricular pressure volume loop relations. The use of<br />
pressure-volume (PV) loop analysis as a means of measuring loadindependent<br />
contractility has largely been restricted to the study<br />
of LV hemodynamics and the interaction between the LV and the<br />
systemic vasculature. 21,25-36 This has primarily been due to geometric<br />
differences between the 2 ventricles and the optimal conductance<br />
properties required for proper volume measurements<br />
and the belief that it is difficult to obtain consistent data using<br />
conductance measurements in the crescent-shaped RV.<br />
Under conditions of normal PAP and RV function, an analysis<br />
of the RV PV loop is somewhat complicated given the crescent<br />
shape of the normal RV (Figure) and the ellipsoid shape of the PV<br />
loop obtained under these conditions. However, under conditions<br />
of even only modestly increased load, the RV changes shape to<br />
one resembling the more spherical LV and allows for measurement<br />
of end-systolic elastance (Ees) and effective arterial elastance<br />
(Ea) as well as the more accurate measurements of indices<br />
of RV systolic and diastolic function as well as RV/PA coupling<br />
(Figure).<br />
The performance of such studies is relatively easy and can be<br />
made in the same acquisition time as making measurements<br />
using FDA-approved equipment. Essentially, all of the currently<br />
used PAH therapies, particularly the phosphodiesterase inhibitors<br />
and endothelin receptor antagonists as well as many of the emerging<br />
experimental therapies (eg, imatinib) have primary—positive<br />
or negative—effects on the myocardium. 37-44 Thus, a study of the<br />
intrinsic contractility of the RV is perhaps the only reliable way to<br />
separate the effects of these therapies on pulmonary arterial systolic<br />
pressure versus the RV myocardium. In that sense, studies<br />
of the RV contractility are not only relevant to the clinical management<br />
of PAH patients but critical for the interpretation of data<br />
from clinical trials as well.<br />
344 Advances in Pulmonary Hypertension
Summary<br />
While traditional resting right heart catheterization techniques<br />
still remain the gold standard for diagnosing pulmonary hypertension<br />
and managing patients on therapy, there are novel techniques<br />
that do not add significant time or risk to the procedure<br />
that may add greatly to our understanding of the RV and the interaction<br />
of the RV with the pulmonary circulation.<br />
References<br />
1. D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary<br />
pulmonary hypertension. Results from a national prospective registry. Ann Intern<br />
Med. 1991;115:343-349.<br />
2. Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart<br />
catheterization procedures in patients with pulmonary hypertension in experienced<br />
centers. J Am Coll Cardiol. 2006;48:2546-2552.<br />
3. Davidson CJ, Bonow RO. Cardiac catheterization. In: Libby P, Bonow RO,<br />
Mann DL, Zipes DP, eds. Braunwald’s Heart Disease: A textbook of cardiovascular<br />
medicine. 8th ed. Philadelphia: Saunders Elsevier; 2007:449.<br />
4. Grossman W B. Pulmonary Hypertension. 3rd ed. Philadelphia: Saunders;<br />
1988.<br />
5. Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment<br />
of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl<br />
12):40S-47S.<br />
6. Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352<br />
(9129):719-725.<br />
7. Hemnes AR, Champion HC. Right heart function and haemodynamic in<br />
pulmonary hypertension. Int J Clin Pract Suppl. 2008 Jul;(160):11-19.<br />
8. Reeves JT, Dempsey JA, Grover RF. Pulmonary circulation during exercise.<br />
In: Weir EK, Reeves JT, eds. Pulmonary Vascular Physiology and Pathophysiology.<br />
1st ed. New York: Marcel Dekker, Inc; 1989:107-135.<br />
9. Brower R, Permutt S. Exercise and the Pulmonary Circulation. In: Whipp<br />
BJ, Wasserman K, eds. Exercise: Pulmonary Physiology and Pathophysiology<br />
in Lung Biology in Health and Disease. <strong>Vol</strong> 52. New York: Marcel Dekker, Inc;<br />
1991:201-221.<br />
10. Ehrsam RE, Perruchoud A, Oberholzer M, Burkart F, Herzog H. Influence<br />
of age on pulmonary haemodynamics at rest and during supine exercise. Clin<br />
Sci (Lond). 1983;65:653-660.<br />
11. Huez S, Brimioulle S, Naeije R, Vachiery JL. Feasibility of routine pulmonary<br />
arterial impedance measurements in pulmonary hypertension. Chest.<br />
2004;125:2121-2128.<br />
12. Kussmaul WG, <strong>No</strong>ordergraaf A, Laskey WK. Right ventricular-pulmonary<br />
arterial interactions. Ann Biomed Eng. 1992;20:63-80.<br />
13. Parmley WW, Tyberg JV, Glantz SA. Cardiac dynamics. Annu Rev Physiol.<br />
1977;39:277-299.<br />
14. Piene H. Pulmonary arterial impedance and right ventricular function.<br />
Physiol Rev. 1986;66:606-652.<br />
15. Castelain V, Herve P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D.<br />
Pulmonary artery pulse pressure and wave reflection in chronic pulmonary<br />
thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol.<br />
2001;37:1085-1092.<br />
16. Ewalenko P, Stefanidis C, Holoye A, Brimioulle S, Naeije R. Pulmonary<br />
vascular impedance vs. resistance in hypoxic and hyperoxic dogs: effects of<br />
propofol and isoflurane. J Appl Physiol. 1993;74:2188-2193.<br />
17. Fourie PR, Coetzee AR. Effect of compliance on a time-domain estimate<br />
of the characteristic impedance of the pulmonary artery during acute pulmonary<br />
hypertension. Med Biol Eng Comput. 1993;31:468-474.<br />
18. Ha B, Lucas CL, Henry GW, Frantz EG, Ferreiro JI, Wilcox BR. Effects of<br />
chronically elevated pulmonary arterial pressure and flow on right ventricular<br />
afterload. Am J Physiol. 1994;267(1 Pt 2):H155-165.<br />
19. Lambermont B, D’Orio V, Gerard P, Kolh P, Detry O, Marcelle R. Time domain<br />
method to identify simultaneously parameters of the windkessel model<br />
applied to the pulmonary circulation. Arch Physiol Biochem. 1998;106:245-<br />
252.<br />
20. Lieber BB, Li Z, Grant BJ. Beat-by-beat changes of viscoelastic and inertial<br />
properties of the pulmonary arteries. J Appl Physiol. 1994;76:2348-2355.<br />
21. O’Rourke MF, Yaginuma T, Avolio AP. Physiological and pathophysiological<br />
implications of ventricular/vascular coupling. Ann Biomed Eng. 1984;12:<br />
119-134.<br />
22. Pagnamenta A, Bouckaert Y, Wauthy P, Brimioulle S, Naeije R. Continuous<br />
versus pulsatile pulmonary hemodynamics in canine oleic acid lung injury.<br />
Am J Respir Crit Care Med. 2000;162(3 Pt 1):936-940.<br />
23. Zuckerman BD, Orton EC, Latham LP, Barbiere CC, Stenmark KR, Reeves<br />
JT. Pulmonary vascular impedance and wave reflections in the hypoxic calf. J<br />
Appl Physiol. 1992;72:2118-2127.<br />
24. Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship<br />
of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial<br />
hypertension. J Am Coll Cardiol. 2006;47:799-803.<br />
25. Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled<br />
systolic-ventricular and vascular stiffening with age: implications for pressure<br />
regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;<br />
32:1221-1227.<br />
26. Cho PW, Levin HR, Curtis WE, et al. Pressure-volume analysis of changes in<br />
cardiac function in chronic cardiomyoplasty. Ann Thorac Surg. 1993;56:38-45.<br />
27. Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic<br />
implications. Heart Fail Rev. 2002;7:51-62.<br />
28. Kass DA. Clinical evaluation of left heart function by conductance catheter<br />
technique. Eur Heart J. 1992;13 (Suppl E):57-64.<br />
29. Kass DA, Midei M, Graves W, Brinker JA, Maughan WL. Use of a conductance<br />
(volume) catheter and transient inferior vena caval occlusion for<br />
rapid determination of pressure-volume relationships in man. Cathet Cardiovasc<br />
Diagn. 1988;15:192-202.<br />
30. Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of<br />
arterial vascular load in humans. Circulation. 1992;86:513-521.<br />
31. Lee WS, Nakayama M, Huang WP, et al. Assessment of left ventricular<br />
end-systolic elastance from aortic pressure-left ventricular volume relations.<br />
Heart Vessels. 2002;16:99-104.<br />
32. Liu CP, Ting CT, Yang TM, et al. Reduced left ventricular compliance in<br />
human mitral stenosis. Role of reversible internal constraint. Circulation.<br />
1992;85:1447-1456.<br />
33. Nussbacher A, Gerstenblith G, O’Connor FC, et al. Hemodynamic effects<br />
of unloading the old heart. Am J Physiol. 1999;277(5 Pt 2):H1863-1871.<br />
34. Pak PH, Kass DA. Assessment of ventricular function in dilated cardiomyopathies.<br />
Curr Opin Cardiol. 1995;10:339-344.<br />
35. O’Rourke MF. Vascular impedance in studies of arterial and cardiac function.<br />
Physiol Rev. 1982;62:570-623.<br />
36. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular<br />
disease in brain and kidney: cause and logic of therapy. Hypertension.<br />
2005;46:200-204.<br />
37. Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates<br />
bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition<br />
of ROS generation and RhoA/Rho kinase activation. Am J Physiol<br />
Lung Cell Mol Physiol. 2008;294:L24-33.<br />
38. Takimoto E, Belardi D, Tocchetti CG, et al. Compartmentalization of cardiac<br />
beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation.<br />
2007;115:2159-2167.<br />
39. Takimoto E, Champion HC, Belardi D, et al. cGMP catabolism by phosphodiesterase<br />
5A regulates cardiac adrenergic stimulation by NOS3-dependent<br />
mechanism. Circ Res. 2005;96:100-109.<br />
40. Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP<br />
phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med.<br />
2005;11:214-222.<br />
41. Nagendran J AS, Gurtu V, Webster L, Ross DB, Rebeyka IM, Michelakis<br />
ED. Phosphodiesterase tType 5 is highly expressed in the hypertrophied human<br />
right ventricle: direct implications for patients with pulmonary hypertension.<br />
Circulation. 2006;114(Suppl):II-667.<br />
42. Allanore Y, Meune C, Vignaux O, Weber S, Legmann P, Kahan A. Bosentan<br />
increases myocardial perfusion and function in systemic sclerosis: a magnetic<br />
resonance imaging and Tissue-Doppler echography study. J Rheumatol.<br />
2006;33:2464-2469.<br />
43. Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacol<br />
Ther. 2006;110:386-414.<br />
44. Packer M, McMurray J, Massie BM, et al. Clinical effects of endothelin receptor<br />
antagonism with bosentan in patients with severe chronic heart failure:<br />
results of a pilot study. J Card Fail. 2005;11:12-20.<br />
Advances in Pulmonary Hypertension 345
Continuing Medical Education Section<br />
Animal Models of Human Severe PAH<br />
Ivan F. McMurtry, PhD<br />
Departments of Pharmacology and<br />
Medicine and Center for Lung Biology<br />
<strong>University</strong> of South Alabama<br />
Mobile, Alabama, USA<br />
Ivan F. McMurtry, PhD<br />
Based on differences in clinical presentation, diagnostic findings,<br />
and response to treatment, human pulmonary hypertension (PH)<br />
has been subdivided into 5 categories. These include 1 :<br />
• Pulmonary arterial hypertension (PAH)<br />
• PH with left-sided heart disease<br />
• PH associated with respiratory disorders and/or hypoxemia<br />
• PH caused by chronic thrombotic and/or embolic disease<br />
• PH caused by miscellaneous other disorders affecting the<br />
pulmonary vasculature.<br />
The PAH category encompasses the idiopathic and familial<br />
forms of PH as well as those that occurr secondarily to several<br />
other diseases or conditions, including connective tissue disease,<br />
congenital systemic-to-pulmonary shunts, HIV infection, portal<br />
hypertension, hemoglobinopathies, and ingestion of drugs and<br />
toxins. This group also includes pulmonary veno-occlusive disease,<br />
pulmonary capillary hemangiomatosis, and persistent pulmonary<br />
hypertension of the newborn.<br />
Address for reprints and other correspondence: Ivan F. McMurtry, PhD, Professor,<br />
Departments of Pharmacology and Medicine and Center for Lung Biology,<br />
<strong>University</strong> of South Alabama, 307 N. <strong>University</strong> Blvd., Mobile, AL<br />
36688; email: ifmcmurtry@usouthal.edu.<br />
Characteristics of PAH<br />
While any form of PH can contribute to patient debilitation and<br />
mortality, PAH is a particularly severe and progressive form that<br />
frequently leads to right heart failure and premature death. 2-4 The<br />
pathogenesis of the increased precapillary pulmonary vascular resistance<br />
(PVR) is generally ascribed to combined effects of vasoconstriction,<br />
arterial wall remodeling, and in situ thrombosis. 5-8<br />
What appears to distinguish PAH from other forms of PH is the<br />
severity of the arteriopathy. Whereas the early phase of PAH has<br />
been described as histologically nonspecific, showing medial hypertrophy<br />
and mild intimal thickening of muscular pulmonary arteries,<br />
the later more progressive stage involves formation of<br />
complex cellular and fibrotic neointimal and plexiform lesions that<br />
obstruct and obliterate medium and small pulmonary arteries and<br />
arterioles. 9-13 This cellular and fibrotic luminal obliteration presumably<br />
accounts for the poor responsiveness of most adult PAH<br />
patients to acute administration of conventional pulmonary vasodilators,<br />
and the irreversibility of the hypertension following<br />
corrective surgery in some PAH patients with congenital heart<br />
diseases. 12-16<br />
Current Treatment of PAH<br />
The goals for the treatment of PAH are to reduce PVR and pulmonary<br />
arterial pressure, and thereby to reverse the pressure overload<br />
of the right ventricle to prevent failure and death. 2,3,17 In<br />
addition to adjunctive therapy with anticoagulants, diuretics, inotropes,<br />
and supplemental oxygen, patients with PAH who are not<br />
candidates for calcium channel blockers are currently treated with<br />
prostacyclin analogs, endothelin-1 receptor blockers, and/or phosphodiesterase<br />
type 5 inhibitors. This treatment improves symptoms<br />
and quality of life, but a recent meta-analysis of several<br />
clinical trials of these agents in patients with severe PAH showed<br />
only moderate reductions in PVR and pulmonary arterial pressure.<br />
18,19 There was no statistically significant decrease in mortality.<br />
These disappointing results do not duplicate those found in<br />
animal studies, which show that these classes of drugs, and numerous<br />
others, largely prevent and in some cases reverse chronic<br />
hypoxia- and monocrotaline-induced PH in rats. 20-23<br />
Classical Animal Models of PH<br />
The limitations of using chronically hypoxic and monocrotaline-injected<br />
rats as models of human severe PAH have been previously<br />
discussed. 22,24-28 The PH in these models is due largely to sustained<br />
vasoconstriction. 29-31 <strong>No</strong>tably, there is no formation of obstructive<br />
intimal lesions in the peripheral pulmonary arteries.<br />
Whether there is loss, rarefaction, of pulmonary microvessels, or<br />
simply impaired filling of these vessels with indicator due to<br />
spasm of upstream hypertensive arteries, is controversial. 31-33 In<br />
any case, it is apparent that preventing or reversing the sustained<br />
constriction and increased muscularization and adventitial thickening<br />
of pulmonary arteries in these 2 rodent models is not equivalent<br />
to “dissolving” the obliterative neointimal and other complex<br />
vascular lesions, and/or reversing unconventional mechanisms of<br />
vasoconstriction, that seemingly account for the high pulmonary<br />
vascular resistance (PVR) in human severe PAH. The same limitations<br />
apply to chronically hypoxic and monocrotaline pyrrole-injected<br />
mice, which typically show even less pulmonary artery<br />
346 Advances in Pulmonary Hypertension
Table. Animal Models That Develop Obstructive, Neointimal<br />
Lesion-Associated Pulmonary Hypertension<br />
Animal Model Cells of Obstructive Lesions<br />
Rat Left pneumonectomy + MCT 53 SMCs<br />
Left pneumonectomy + MCT +<br />
MCT in younger animals 63<br />
ET B<br />
receptor deficient +MCT 45<br />
Sugen 5416 + chronic hypoxia 57<br />
Athymic and Sugen 5416 58<br />
ECs in perivascular lesions<br />
ECs and SMCs<br />
ECs<br />
ECs and B-lymphocytes with<br />
perivascular inflammatory cells<br />
Mouse S100A4/Mts1 over expression 43 SMCs with perivascular inflammatory<br />
cells<br />
S100A4/Mts1 over expression<br />
exacerbated by infection with<br />
M1γherpesvirus 68 54<br />
Lung-specific IL-6 over<br />
expression exacerbated by<br />
chronic hypoxia 55<br />
Repeated inhalation of ?<br />
Stachybotrys chartarum spores 51<br />
SMCs with perivascular<br />
inflammatory cells<br />
ECs and T-lymphocytes with<br />
perivascular inflammatory cells<br />
Beagle Dehydromonocrotaline 64 ?<br />
Macaque SHIV-nef infection 65 ECs and SMCs with lymphatic infiltration<br />
Calf Aorta-pulmonary artery ?<br />
anastamosis 66<br />
Piglet Aorta-pulmonary artery ?<br />
anastamosis 67<br />
remodeling than rats. 34-35 Although chronically hypoxic bovine<br />
calves and fawn-hooded rats develop severe PH with marked medial<br />
and adventitial thickening of pulmonary arteries, there are no<br />
reports of obliterative neointimal lesions in the resistance arteries<br />
of these models. 36-40<br />
Animal Models of PAH<br />
Studies of the classic chronically hypoxic and monocrotaline-injected<br />
models of PH have produced an abundance of important information<br />
on cellular and molecular mechanisms of pulmonary<br />
vasoconstriction and medial and adventitial remodeling. However,<br />
investigators who evaluate new therapeutic strategies for severe<br />
PAH should consider using more recent animal models of obstructive,<br />
neointimal lesion-associated PH. At least 10 different<br />
rodent models of peripheral pulmonary artery neointimal lesion<br />
formation have now been described and studied (Table). 41-67<br />
These models develop PH accompanied by formation of obstructive<br />
cellular lesions in the lumen of small pulmonary arteries<br />
and arterioles, in addition to increased medial muscularization<br />
of proximal and distal pulmonary arteries. The proliferative neointimal<br />
lesions are variously reported to comprise phenotypically abnormal<br />
smooth muscle cells, endothelial cells, cells that express<br />
both endothelial and smooth muscle cell markers, and inflammatory<br />
cells. This is similar to the cellular heterogeneity reported in<br />
the lesions of human forms of PAH. 9-11,13<br />
The lesions in some of these models are<br />
considered to resemble the plexiform lesions<br />
of human PAH. 43,54,55,57,63<br />
In addition to the rodent models,<br />
PAH arteriopathy has also been observed<br />
in young beagles who have been exposed<br />
to dehydromonocrotaline (an endothelial<br />
cell-toxic metabolite of monocrotaline),<br />
macaques infected with SHIV-nef (a<br />
chimeric viral construct containing the<br />
HIV nef gene in a simian immunodeficiency<br />
virus backbone), and calves and<br />
piglets with anastamosis of the left lower<br />
lobe pulmonary artery to the aorta. 64-67<br />
<strong>No</strong>t all studies of aortopulmonary shunts<br />
in young pigs, however, have found<br />
formation of peripheral neointimal lesions.<br />
68<br />
Treatment of Animal Models<br />
With regard to using the rodent models of<br />
neointimal PAH to identify more effective<br />
therapeutic drugs, the 3-hydroxy-3-<br />
methyl-glutaryl-CoA (HMG-CoA) reductase<br />
inhibitor simvastatin has been found<br />
to attenuate the development of PAH<br />
and, in a more clinically relevant experiment,<br />
to reverse the established disease<br />
and promote survival in left pneumonectomized<br />
plus monocrotaline-injected<br />
rats. 48,50 Similar results, albeit not with<br />
complete reversal of hypertension and<br />
neointimal lesions, were seen with triptolide<br />
treatment, an agent that has antitumor,<br />
antiangiogenic, and antiproliferative<br />
effects; rapamycin, an immunosuppressant<br />
and antiproliferative agent; and the naturally occurring<br />
steroid hormone dehydroepiandrosterone (DHEA). 42,44,49,61<br />
Although DHEA treatment did not completely reverse the PAH, it<br />
was associated with 100% survival as compared to 30% in DHEAuntreated<br />
rats.<br />
In the Sugen 5416 (vascular endothelial growth factor [VEGF]<br />
receptor blocker) -injected plus chronic hypoxia-exposed rat<br />
model, treatment with the bradykinin antagonist B9430, the caspase<br />
inhibitor Z-Asp-2,6-dichlorobenzoyloxymethylketone, or the<br />
anti-cancer drug sorafenib prevented development of the<br />
PAH. 47,56,57 With respect to reversal studies, treatment with the<br />
bradykinin receptor agonist B9972 or simvastatin arrested progression<br />
of the established PAH but did not reverse the hypertension<br />
or neointimal lesions. 59,60 Several other drugs with a<br />
variety of actions, including the anticancer drugs cyclophosphamide<br />
and paclitaxel, the angiotensin-converting enzyme inhibitor<br />
lisinopril, the angiotensin II type 1 receptor blocker<br />
irbesartan, the bradykinin antagonist B9430, the antiangiogenic<br />
agent thalidomide, the peroxisome proliferator-actived receptor-γ<br />
agonist PGJ2, and the calcium channel blocker nifedipine, failed<br />
to arrest progression of the PAH. <strong>No</strong> reversal experiment with sorafenib<br />
has been reported. Even so, clinical trials with both simvastatin<br />
and sorafenib in PAH are currently under way.<br />
Advances in Pulmonary Hypertension 347
Conclusion<br />
Although it is not clear how closely any of the neointimal animal<br />
models mimic the multifactorial pathobiology of human PAH, it is<br />
probable that they will provide insights into pathological cellular<br />
and molecular signaling pathways and potentially effective therapies<br />
that would not be revealed or rigorously tested in the classic<br />
chronically hypoxic and monocrotaline-injected models.<br />
Another point is that while prevention studies may provide useful<br />
information, the more clinically relevant experiment is to determine<br />
if the treatment reverses the neointimal arteriopathy and hypertension<br />
once they are well established. Finally, it needs to be<br />
noted that even if a novel drug or therapeutic strategy is found to<br />
effectively reverse PAH, and/or prevent right ventricular failure<br />
and death, in one or more of the animal models, that doesn’t necessarily<br />
mean it will work in the human forms of PAH. The cellular<br />
and molecular pathogenesis of obstructive vascular lesions,<br />
and the mechanisms of right ventricular dysfunction, that develop<br />
over a few weeks in the animal models may not duplicate that<br />
which occurs over months or years in human PAH. Careful and<br />
rigorous clinical trials will be required to establish the safety and<br />
efficacy of any new therapy in patients. 69<br />
References<br />
1. Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary<br />
hypertension. J Am Coll Cardiol. 2004;43:5S-12S.<br />
2. Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension.<br />
Coron Artery Dis. 2005;16:13-18.<br />
3. Hemnes AR, Champion HC. Right heart function and haemodynamics in<br />
pulmonary hypertension. Int J Clin Pract Suppl. 2008:11-19.<br />
4. Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and<br />
failure: Report of a national heart, lung, and blood institute working group on<br />
cellular and molecular mechanisms of right heart failure. Circulation.<br />
2006;114:1883-1891.<br />
5. Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension.<br />
J Mol Cell Cardiol. 2008;44:14-30.<br />
6. Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology<br />
of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S-24S.<br />
7. Rabinovitch M. Pathobiology of pulmonary hypertension. Annu Rev Pathol.<br />
2007;2:369-399.<br />
8. Widlitz A, Barst RJ. Pulmonary arterial hypertension in children. Eur Respir<br />
J. 2003;21:155-176.<br />
9. Mooi WJ, Grungerg K. Histopatholgy of pulmonary hypertensive diseases.<br />
Current Diag Pathol. 2006;12:429-440.<br />
10. Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies<br />
in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25S-32S.<br />
11. Tuder RM, Marecki JC, Richter A, et al. Pathology of pulmonary hypertension.<br />
Clin Chest Med. 2007;28:23-42, vii.<br />
12. Wagenvoort CA. Open lung biopsies in congenital heart disease for evaluation<br />
of pulmonary vascular disease: predictive value with regard to corrective<br />
operability. Histopathology. 1985;9:417-436.<br />
13. Yi ES, Kim H, Ahn H, et al. Distribution of obstructive intimal lesions and<br />
their cellular phenotypes in chronic pulmonary hypertension. A morphometric<br />
and immunohistochemical study. Am J Respir Crit Care Med. 2000;162:<br />
1577-1586.<br />
14. Palevsky HI, Schloo BL, Pietra GG, et al. Primary pulmonary hypertension.<br />
Vascular structure, morphometry, and responsiveness to vasodilator agents.<br />
Circulation. 1989;80:1207-1221.<br />
15. Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel<br />
blockers in idiopathic pulmonary arterial hypertension. Circulation.<br />
2005;111:3105-3111.<br />
16. Levy M, Maurey C, Celermajer DS, et al. Impaired apoptosis of pulmonary<br />
endothelial cells is associated with intimal proliferation and irreversibility of<br />
pulmonary hypertension in congenital heart disease. J Am Coll Cardiol.<br />
2007;49:803-810.<br />
17. Rich S. The current treatment of pulmonary arterial hypertension: time to<br />
redefine success. Chest. 2006;130:1198-1202.<br />
18. Macchia A, Marchioli R, Marfisi R, et al. A meta-analysis of trials of pulmonary<br />
hypertension: a clinical condition looking for drugs and research<br />
methodology. Am Heart J. 2007;153:1037-1047.<br />
19. Rich S. The value of approved therapies for pulmonary arterial hypertension.<br />
Am Heart J. 2007;153:889-890.<br />
20. Campian ME, Hardziyenka M, Michel MC, et al. How valid are animal<br />
models to evaluate treatments for pulmonary hypertension? Naunyn Schmiedebergs<br />
Arch Pharmacol. 2006;373:391-400.<br />
21. Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: A target for therapeutic<br />
intervention in pulmonary hypertension. Pharmacol Ther. 2001;92:1-<br />
20.<br />
22. Marsboom GR, Janssens SP. Models for pulmonary hypertension. Drug<br />
Discovery Today: Disease Models. 2004;1:289-296.<br />
23. Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational<br />
priorities in pulmonary arterial hypertension. Circulation. 2008;118:<br />
1486-1495.<br />
24. Bauer NR, Moore TM, McMurtry IF. Rodent models of pah: Are we there<br />
yet? Am J Physiol Lung Cell Mol Physiol. 2007;293:L580-582.<br />
25. Heath D. The rat is a poor animal model for the study of human pulmonary<br />
hypertension. Cardioscience. 1992;3:1-6.<br />
26. Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling:<br />
A model for what human disease? J Clin Invest. 2000;106:733-738.<br />
27. White RJ. Pulmonary arterial hypertension: Building a better mouse trap<br />
for 2010. Drug Discovery Today: Therapeutic Strategies. 2004;1:351-359.<br />
28. Zaiman A, Fijalkowska I, Hassoun PM, et al. One hundred years of research<br />
in the pathogenesis of pulmonary hypertension. Am J Respir Cell Mol<br />
Biol. 2005;33:425-431.<br />
29. McMurtry IF, Gebb SA, Jones PL. Pulmonary vascular remodeling. In: Laurent<br />
GJ, Shapiro SD,eds. Encyclopedia of Respiratory Medicine. New York:<br />
Academic Press; 2006:. 591-599.<br />
30. Oka M, Fagan KA, Jones PL, et al. Therapeutic potential of rhoa/rho kinase<br />
inhibitors in pulmonary hypertension. Br J Pharmacol. 2008;155:444-<br />
454.<br />
31. Tenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction<br />
in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res.<br />
2005;97:95-98.<br />
32. McLoughlin P, McMurtry I. Counterpoint: chronic hypoxia-induced pulmonary<br />
hypertension does not lead to loss of pulmonary vasculature. J Appl<br />
Physiol. 2007;103:1451-1453; discussion 1453-1454.<br />
33. Rabinovitch M, Chesler N, Molthen RC. Point:Counterpoint: chronic hypoxia-induced<br />
pulmonary hypertension does/does not lead to loss of pulmonary<br />
vasculature. J Appl Physiol. 2007;103:1449-1451.<br />
34. Dumitrascu R, Koebrich S, Dony E, et al. Characterization of a murine<br />
model of monocrotaline pyrrole-induced acute lung injury. BMC Pulm Med.<br />
2008;8:25.<br />
35. Hoshikawa Y, Nana-Sinkam P, Moore MD, et al. Hypoxia induces different<br />
genes in the lungs of rats compared with mice. Physiol Genomics. 2003;<br />
12:209-219.<br />
36. Durmowicz AG, Orton EC, Stenmark KR. Progressive loss of vasodilator responsive<br />
component of pulmonary hypertension in neonatal calves exposed<br />
to 4570 m. Am J Physiol. 1993;265:H2175-2183.<br />
37. Jaenke RS, Alexander AF. Fine structural alterations of bovine peripheral<br />
pulmonary arteries in hypoxia-induced hypertension. Am J Pathol. 1973;73:<br />
377-398.<br />
38. Bonnet S, Michelakis ED, Porter CJ, et al. An abnormal mitochondrial-hypoxia<br />
inducible factor-1alpha-kv channel pathway disrupts oxygen sensing and<br />
triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to<br />
human pulmonary arterial hypertension. Circulation. 2006;113:2630-2641.<br />
39. Nagaoka T, Gebb SA, Karoor V, et al. Involvement of rhoa/rho kinase signaling<br />
in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol.<br />
2006;100:996-1002.<br />
40. Sato K, Webb S, Tucker A, et al. Factors influencing the idiopathic development<br />
of pulmonary hypertension in the fawn hooded rat. Am Rev Respir<br />
Dis. 1992;145:793-797.<br />
41. Achcar RO, Demura Y, Rai PR, et al. Loss of caveolin and heme oxygenase<br />
expression in severe pulmonary hypertension. Chest. 2006;129:696-705.<br />
42. Faul JL, Nishimura T, Berry GJ, et al. Triptolide attenuates pulmonary arterial<br />
hypertension and neointimal formation in rats. Am J Respir Crit Care<br />
Med. 2000;162:2252-2258.<br />
43. Greenway S, van Suylen RJ, Du Marchie Sarvaas G, et al. S100a4/mts1<br />
produces murine pulmonary artery changes resembling plexogenic arteriopathy<br />
and is increased in human plexogenic arteriopathy. Am J Pathol.<br />
2004;164:253-262.<br />
44. Homma N, Nagaoka T, Karoor V, et al. Involvement of rhoa/rho kinase signaling<br />
in protection against monocrotaline-induced pulmonary hypertension in<br />
pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell<br />
Mol Physiol. 2008;295:L71-78.<br />
348 Advances in Pulmonary Hypertension
45. Ivy DD, McMurtry IF, Colvin K, et al. Development of occlusive neointimal<br />
lesions in distal pulmonary arteries of endothelin b receptor-deficient rats: A<br />
new model of severe pulmonary arterial hypertension. Circulation. 2005;111:<br />
2988-2996.<br />
46. Merklinger SL, Wagner RA, Spiekerkoetter E, et al. Increased fibulin-5<br />
and elastin in s100a4/mts1 mice with pulmonary hypertension. Circ Res.<br />
2005;97:596-604.<br />
47. Moreno-Vinasco L, Gomberg-Maitland M, Maitland ML, et al. Genomic<br />
assessment of a multikinase inhibitor, sorafenib, in a rodent model of pulmonary<br />
hypertension. Physiol Genomics. 2008;33:278-291.<br />
48. Nishimura T, Faul JL, Berry GJ, et al. Simvastatin attenuates smooth muscle<br />
neointimal proliferation and pulmonary hypertension in rats. Am. J. Respir.<br />
Crit. Care Med. 2002;166:1403-1408.<br />
49. Nishimura T, Faul JL, Berry GJ, et al. 40-o-(2-hydroxyethyl)-rapamycin<br />
attenuates pulmonary arterial hypertension and neointimal formation in rats.<br />
Am. J. Respir. Crit. Care Med. 2001;163:498-502.<br />
50. Nishimura T, Vaszar LT, Faul JL, et al. Simvastatin rescues rats from fatal<br />
pulmonary hypertension by inducing apoptosis of neointimal smooth muscle<br />
cells. Circulation. 2003;108:1640-1645.<br />
51. Ochiai E, Kamei K, Watanabe A, et al. Inhalation of stachybotrys chartarum<br />
causes pulmonary arterial hypertension in mice. Int J Exp Pathol.<br />
2008;89:201-208.<br />
52. Oka M, Homma N, Taraseviciene-Stewart L, et al. Rho kinase-mediated<br />
vasoconstriction is important in severe occlusive pulmonary arterial hypertension<br />
in rats. Circ Res. 2007;100:923-929.<br />
53. Okada K, Tanaka Y, Bernstein M, et al. Pulmonary hemodynamics modify<br />
the rat pulmonary artery response to injury: a neointimal model of pulmonary<br />
hypertension. Am J Pathol. 1997;151:1019-1025.<br />
54. Spiekerkoetter E, Alvira CM, Kim Y-M, et al. Reactivation of {gamma}hv68<br />
induces neointimal lesions in pulmonary arteries of s100a4/mts1-overexpressing<br />
mice in association with degradation of elastin. Am J Physiol Lung<br />
Cell Mol Physiol. 2008;294:L276-289.<br />
55. Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression<br />
induces pulmonary hypertension. Circ Res. 2008.<br />
56. Taraseviciene-Stewart L, Gera L, Hirth P, et al. A bradykinin antagonist and<br />
a caspase inhibitor prevent severe pulmonary hypertension in a rat model. Can<br />
J Physiol Pharmacol. 2002;80:269-274.<br />
57. Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. Inhibition of the vegf<br />
receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary<br />
endothelial cell proliferation and severe pulmonary hypertension.<br />
FASEB J. 2001;15:427-438.<br />
58. Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, et al. Absence of t<br />
cells confers increased pulmonary arterial hypertension and vascular remodeling.<br />
Am J Respir Crit Care Med. 2007;175:1280-1289.<br />
59. Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. Simvastatin<br />
causes endothelial cell apoptosis and attenuates severe pulmonary hypertension.<br />
Am J Physiol Lung Cell Mol Physiol. 2006;291:L668-676.<br />
60. Taraseviciene-Stewart L, Scerbavicius R, Stewart JM, et al. Treatment of<br />
severe pulmonary hypertension: a bradykinin receptor 2 agonist b9972 causes<br />
reduction of pulmonary artery pressure and right ventricular hypertrophy. Peptides.<br />
2005;26:1292-1300.<br />
61. Vaszar LT, Nishimura T, Storey JD, et al. Longitudinal transcriptional analysis<br />
of developing neointimal vascular occlusion and pulmonary hypertension<br />
in rats. Physiol Genomics. 2004;17:150-156.<br />
62. West J, Harral J, Lane K, et al. Mice expressing bmpr2r899x transgene<br />
in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell<br />
Mol Physiol. 2008;295:L744-755.<br />
63. White RJ, Meoli DF, Swarthout RF, et al. Plexiform-like lesions and increased<br />
tissue factor expression in a rat model of severe pulmonary arterial hypertension.<br />
Am J Physiol Lung Cell Mol Physiol. 2007;293:L583-590.<br />
64. Gust R, Schuster DP. Vascular remodeling in experimentally induced subacute<br />
canine pulmonary hypertension. Exp Lung Res. 2001;27:1-12.<br />
65. Marecki JC, Cool CD, Parr JE, et al. Hiv-1 nef is associated with complex<br />
pulmonary vascular lesions in shiv-nef-infected macaques. Am J Respir Crit<br />
Care Med. 2006;174:437-445.<br />
66. Fasules JW, Tryka F, Chipman CW, et al. Pulmonary hypertension and arterial<br />
changes in calves with a systemic-to-left pulmonary artery connection.<br />
J Appl Physiol. 1994;77:867-875.<br />
67. Bousamra M, 2nd, Rossi R, Jacobs E, et al. Systemic lobar shunting induces<br />
advanced pulmonary vasculopathy. J Thorac Cardiovasc Surg. 2000;<br />
120:88-98.<br />
68. Wauthy P, Abdel Kafi S, Mooi WJ, et al. Inhaled nitric oxide versus prostacyclin<br />
in chronic shunt-induced pulmonary hypertension. J Thorac Cardiovasc<br />
Surg. 2003;126:1434-1441.<br />
69. Ghofrani HA, Wilkins MW, Rich S. Uncertainties in the diagnosis and treatment<br />
of pulmonary arterial hypertension. Circulation. 2008;118:1195-1201.<br />
The Pulmonary Hypertension Association<br />
presents the 2009 PH Resource Network Symposium<br />
Leading Progress, r<br />
Creating Partnerships:<br />
rships:<br />
Empowering the Interdisciplinary ry PH Team<br />
September 24 – 26, 2009<br />
Hyatt Regency Crystal City in Arlington, VA<br />
Sessions on September 25 th and 26 th will feature topics such as...<br />
Diagnostic interpretation<br />
PH and related conditions<br />
REVEAL outcomes and new areas of research<br />
Recent advances in treatment, diagnosis and care<br />
Clinical practice issues<br />
...and more<br />
Advocacy Day on September 24 th will include a brief training<br />
followed by small group meetings at the Capitol with your<br />
representatives in Congress.<br />
CNE, CEU & CPE credits will be available<br />
Registration for this unique event will open March 2009.<br />
Visit www.<strong>PHA</strong>ssociation.org/PHRN/Symposium<br />
for the latest updates on registration, speakers and topics.<br />
To MDs:<br />
Please pass on to nurses and<br />
other allied health professionals sionals to make sure that the latest advances<br />
in the care and<br />
treatment of PH patients<br />
are incorporated into your practice!
Connective Tissue Disease-Associated<br />
Pulmonary Hypertension<br />
Christopher P. Denton PhD, FRCP<br />
Professor of Experimental Rheumatology<br />
Royal Free Campus, <strong>University</strong> College London<br />
Head of Scleroderma Service, Royal Free Hospital<br />
London, UK<br />
I was very excited to see a whole issue of Advances in Pulmonary<br />
Hypertension (<strong>Vol</strong>. 7, <strong>No</strong>. 2, Summer 2008) devoted to<br />
connective tissue disease associated pulmonary hypertension<br />
(PH). As a practicing rheumatologist in a center that manages<br />
a large cohort of more than 1000 scleroderma cases, and a<br />
center that benefits from having a pulmonary hypertension centre<br />
embedded within it, all of the topics covered in this issue<br />
were very relevant.<br />
Scleroderma has the highest frequency of pulmonary hypertension<br />
of any of the rheumatic disease and is especially challenging<br />
to manage as cases often have co-morbidity that affects<br />
assessment and may lead to poor outcome. There are also particular<br />
challenges for diagnosis and assessment. All of these<br />
points were highlighted and I was especially pleased to see the<br />
emphasis that was put on autoantibodies as useful tests is<br />
assessing risk of developing pulmonary arterial hypertension<br />
(PAH) in scleroderma. Less invasive tests focused on at-risk<br />
Address for reprints and other correspondence: Christopher P. Denton, PhD<br />
FRCP, Professor of Experimental Rheumatology, Centre for Rheumatology,<br />
Royal Free Hospital, London,NW3 2QG. email: denton@rfhsm.ac.uk<br />
groups will be essential for timely detection and treatment of<br />
PAH in connective tissue disease.<br />
Scleroderma and the antiphospholipid syndromes were<br />
very clearly reviewed and the articles offered clear insight into<br />
the likely differences in frequency of PAH in these diseases<br />
and also the potential contribution of thrombosis to contribute.<br />
Being mindful of pathogenic mechanisms is likely to underpin<br />
better therapy in connective tissue disease associated<br />
pulmonary hypertension. One of the challenges faced daily in<br />
managing connective tissue disease associated PAH is the<br />
co-existence of interstitial lung fibrosis. The degree to which<br />
many patients should have PAH or PH secondary to lung fibrosis<br />
remains a topic of considerable debate and I was interested<br />
to see how this challenge was tackled in some of the major<br />
centres in the USA.<br />
Without doubt, the most engaging and relevant part of this<br />
excellent issue was the round table discussion. Almost all of<br />
the important points raised are as relevant to practice in the<br />
UK and other European centres as they are in USA. It is clear<br />
from the answers to many key questions that this whole topic<br />
requires more research and better information. However, with<br />
exciting projects such as <strong>PHA</strong>ROS for scleroderma and complementary<br />
exercises ongoing in Europe, I was left with optimism<br />
about clinical practice for patients with connective tissue disease-associated<br />
pulmonary hypertension, even if the outcomes<br />
for many patients do not seem to be as good as in idiopathic<br />
PAH.<br />
In conclusion, there are more similarities than differences<br />
in the approach to PAH in Europe and the USA based upon<br />
these articles. This is a testament to the strong international<br />
collaboration in clinical trials and educational programs that<br />
exist in the fields of connective tissue disease and pulmonary<br />
hypertension.
Self-Assessment Examination<br />
See answer key on next page<br />
1. Chronically hypoxia is a frequently used model for PH.<br />
All of the following are features of chronically hypoxic<br />
animal models except<br />
a. Right ventricular hypertrophy<br />
b. Occlusive intimal vascular lesions<br />
c. Medial hypertrophy<br />
d. Thrombosis<br />
2. True or false: The tat protein is implicated in the<br />
pulmonary vascular abnormalities seen in the Simian<br />
HIV infected macaques.<br />
a. True<br />
b. False<br />
3. Which statement is FALSE:<br />
a. Most studies using animal models of PH are<br />
prevention models.<br />
b. Right heart failure is a common feature of animal<br />
models of PH.<br />
c. PH models using mice have relatively less vascular<br />
remodeling compared to other species.<br />
4. Metabolic syndrome is defined by all of the following<br />
abnormalities except:<br />
a. Diabetes<br />
b. Hypertension<br />
c. Hyperlididemia<br />
d. Obesity<br />
7. Poor prognostic signs in exercise right heart catheterization<br />
include all of the following EXCEPT:<br />
a. Increased cardiac output<br />
b. Decrease in pulmonary vascular resistance<br />
c. Angina<br />
d. Presyncopal symptoms or frank syncope<br />
8. Conventional hemodynamic assessment during right<br />
heart catheterization does not allow for consideration of<br />
which of the following:<br />
a. Left-sided filling pressures<br />
b. Pulmonary vascular resistance<br />
c. Pulsatile stress<br />
ERRATUM<br />
In the last issue of Advances in Pulmonary Hypertension<br />
there was an error on the posttest.<br />
1. The answer should have been E (B and C), but<br />
this is a typo as it says “C and D.” The correct<br />
answer is either B or C. A is wrong, therefore D is<br />
wrong.<br />
5. “Diabetic cardiomyopathy” is defined as impaired<br />
ventricular function in diabetics with:<br />
a. Hypertension<br />
b. Ischemic heart disease<br />
c. Both of the above<br />
d. Neither of the above<br />
6. Ventricular dysfunction in patients with metabolic<br />
syndrome is associated with abnormalities in which of<br />
the following subcellular organelles:<br />
a. Nucleus<br />
b. Golgi apparatus<br />
c. Mitochondria<br />
d. Caveoli<br />
Advances in Pulmonary Hypertension 351
ACCP Update and New Treatments for<br />
PAH Project # 406602<br />
Individuals wishing CME credit for this self-study activity<br />
should read the text, answer the self-assessment examination<br />
questions, complete the form below,* and send<br />
by US mail or fax to the following address by February 1,<br />
2010. You should receive a score of 70% or higher for<br />
CME credit. Your test will be scored and your participation<br />
will be entered into the CME records at the<br />
<strong>University</strong> of Michigan Medical School.<br />
Office of Continuing Medical Education<br />
Attn: Pamela Little<br />
Towsley Center—1500 East Medical Center Drive<br />
<strong>University</strong> of Michigan Medical School<br />
Ann Arbor, MI 48109<br />
Fax: (734) 936-1641<br />
Your certificate will be mailed within 3 weeks of receipt<br />
of request.<br />
Evaluation of CME Activity (see page 330)<br />
Poor Satisfactory Excellent<br />
1. Extent to which objectives were met<br />
1 2 3 4 5<br />
2. Potential impact on your practice<br />
1 2 3 4 5<br />
3. Avoidance of commercial bias or influence<br />
1 2 3 4 5<br />
4. Your overall evaluation of this self-study activity<br />
1 2 3 4 5<br />
Self-Assessment Answer Key<br />
Circle one correct answer<br />
Additional comments about this self-study activity:<br />
1. a b c d 6. a b c d<br />
2. a b 7. a b c d<br />
3. a b c 8. a b c<br />
4. a b c d<br />
5. a b c d<br />
Name<br />
Degree(s)<br />
Specialty<br />
(Please print)<br />
Suggestions for future topics:<br />
Street<br />
City<br />
State<br />
Zip<br />
E-mail Address<br />
Date Test Completed<br />
Check number of CME credits requested<br />
■ 1.0 ■ 1.5 ■ 2.0<br />
* Self-assessment examination may also be<br />
completed online at: http://cme.med.umich.edu<br />
Signature<br />
352 Advances in Pulmonary Hypertension
Pulmonary Hypertension Roundtable<br />
<strong>PHA</strong> Scientific Sessions Provide a Great<br />
Window Into Treatment Breakthroughs<br />
While Inspiring Physicians and Patients Alike<br />
Karen Fagan, MD<br />
Todd Bull, MD<br />
Ivan F. McMurtry, PhD<br />
Omar A. Minai, MD<br />
This roundtable discussion, reviewing the proceedings<br />
from the Pulmonary Hypertension Association’s Eighth<br />
International PH Conference and Scientific Sessions<br />
in Houston in 2008, was moderated by Karen Fagan,<br />
MD, Chief, Division of Pulmonary and Critical Care<br />
Medicine, <strong>University</strong> of South Alabama, Mobile, Alabama.<br />
It included Todd Bull, MD, Associate Professor<br />
of Medicine, Division of Pulmonary Sciences and Critical<br />
Care Medicine, <strong>University</strong> of Colorado Health Sciences<br />
Center, Aurora, Colorado; Ivan F. McMurtry, PhD,<br />
Professor, Department of Pharmacology, <strong>University</strong> of<br />
South Alabama School of Medicine, Mobile, Alabama;<br />
and Omar A. Minai, MD, Staff Physician in the Department<br />
of Pulmonary, Allergy, and Critical Care Medicine<br />
and the Lung Transplant Center at the Cleveland<br />
Clinic, Cleveland, Ohio.<br />
Dr Fagan: The first thing I wanted<br />
to do is to thank you for attending<br />
the Scientific Sessions. As you<br />
know, 2008 was the third Scientific<br />
Sessions that were held in<br />
conjunction with the <strong>PHA</strong> (Pulmonary<br />
Hypertension Association)<br />
International Conference meeting.<br />
The ultimate goal of the Sessions<br />
was to provide the medical professionals<br />
who volunteer their time<br />
during the medically led sessions<br />
and the patient and family oriented<br />
sessions an opportunity to<br />
hear state-of-the-art scientific<br />
speakers, and interact with their<br />
peers before they’re put to work in the International<br />
Conference. I am a little biased because I chaired the<br />
2008 Scientific Sessions committee; I think it went<br />
well.<br />
I think we were successful in bringing together a<br />
diverse crowd and I’d like to hear what your impression<br />
was of what you heard and enjoyed about the<br />
meeting.<br />
Dr Minai: The <strong>PHA</strong> International Conference provides<br />
a great opportunity for physicians to hear lectures on<br />
cutting edge research, to network with other physicians<br />
who may be doing similar work, and to discuss ideas<br />
that later blossom into novel studies. To me this is a<br />
unique meeting in that it is the only one in our field<br />
“The <strong>PHA</strong> International<br />
Conference provides<br />
a great opportunity for<br />
physicians to hear lectures<br />
on cutting edge<br />
research, to network<br />
with other physicians who may be<br />
doing similar work, and to discuss<br />
ideas that later blossom into novel<br />
studies. To me this is a unique meeting<br />
in that it is the only one in our<br />
field that brings patients and physicians<br />
together.”–Dr Minai<br />
that brings patients and physicians together. This experience<br />
serves to “humanize” the disease for professionals<br />
who focus on basic science research and don’t<br />
have the opportunity to meet patients. In addition, this<br />
meeting provides a unique opportunity to the greater<br />
pulmonary hypertension (PH) community to recognize<br />
the achievements and breakthroughs in this field and<br />
to recognize and honor people who give of their precious<br />
time and other resources to the cause of PH. The meeting<br />
allows us to truly see the breadth of the true of impact<br />
of PH on patients, their caregivers, and families.<br />
Dr Bull: The Scientific Sessions have become a part<br />
of the Conference that I enjoy thoroughly. The first<br />
meeting I attended was one of the earlier forays into<br />
this and I think it has progressed beautifully to date.<br />
The most recent Scientific Session was fantastic and<br />
built beautifully on the previous<br />
sessions. A key strength is bringing<br />
speakers outside the PH<br />
community to the meeting to talk<br />
about new ideas and directions<br />
to consider in PH. For those of<br />
us in the PH community, we<br />
have a general understanding of<br />
what everyone’s working on and<br />
of course are excited to hear<br />
about new directions they’re taking<br />
with their own work. But, this<br />
meeting allows the opportunity to<br />
stimulate new thoughts and new<br />
directions.<br />
This year I particularly enjoyed<br />
the pharmacogenomics discussion. Where the<br />
field of pharmacogenomics has arisen from was fascinating<br />
not just from a historical perspective but also<br />
where we may take it in the years to come. It really<br />
tied in well to some of the work that Ray Benza has<br />
been working on, and where we’d like to take this from<br />
a PH standpoint. Quite frankly it transcends more than<br />
just PH and the Dick Weinshilboum approach<br />
was great. I also really enjoyed Hunter Champion’s talk<br />
on the molecular basis of right ventricular (RV) dysfunction.<br />
Dr Minai: Hunter Champion’s talk about the molecular<br />
basis of RV dysfunction really stood out to me. Even<br />
though PH is a disease of the pulmonary vasculature,<br />
Advances in Pulmonary Hypertension 353
the real cause of most morbidity and mortality in our patients is<br />
RV (dys)function. I thought Hunter did a great job of delivering the<br />
message while making it understandable and interesting to the<br />
general audience which included not only basic scientists but clinicians<br />
and patients as well.<br />
Dr Fagan: I also thought that the pharmacogenomics talk by<br />
Dr Weinshilboum was great. He was able to show how pharmacogenomics<br />
doesn’t just have the promise of impacting therapy in<br />
a wide range of diseases, he actually showed concrete examples<br />
of the role of this technology in patient care today. I don’t know<br />
how far into the future we are until we have this type of personalized<br />
medicine in PH, but it certainly raised the hope that maybe<br />
through pharmacogenomics we’ll understand a little bit more<br />
about which patients should be on which initial therapies and<br />
which patients may not respond even to combination treatments.<br />
I think that the hopefulness and the potential promise of this line<br />
of investigation is totally exciting.<br />
Dr Bull: I agree, and I think all of us tell the<br />
patient after we complete their evaluation, we<br />
will pick a therapy. A patient will ask, “Is this<br />
going to work?” And our answer is, “We’re<br />
going to have to wait and see.” And we can<br />
look at the odds and tell you what we think is<br />
going to happen. Of course at this juncture<br />
really honestly we don’t know. We’ve all had<br />
patients who have significant improvements<br />
with a phosphodiesterase inhibitor, for example,<br />
and others it does not seem to touch.<br />
There’s got to be something to that. We’re just<br />
not smart enough yet to know who will benefit<br />
from what drug up front. In the future,<br />
pharmacogemonics may give us some insight.<br />
It is my experience that when I consider therapy,<br />
especially oral therapy, that my recommendations<br />
are more based on toxicity then<br />
efficiacy, ie, this treatment potentially could cause this problem<br />
as opposed to this, may really help. Pharmacogenomics may help<br />
in both areas to identify persons with expected benefit as well as<br />
persons with higher likelihood of toxicity.<br />
Dr Minai: Karen and Todd, I completely agree. When we look at<br />
clinical trials of oral medications in PH, we find that several medications<br />
have very similar overall efficacy in a cluster of patients<br />
and we tend to focus on the mean or average response rate. However,<br />
within each study population there are patients who respond<br />
extremely well, ie, much better than average and those that don’t<br />
respond at all to the same medication. Currently, we lack a reliable<br />
method of identifying these subgroups with any degree of accuracy.<br />
The patient who responds poorly to one medication may<br />
respond very well to another medication. Pharmacogenomics holds<br />
the promise of helping us match the patient with the right drug up<br />
front. That would be a great benefit to physicians in choosing therapy<br />
and to patients in this rapidly progressing disease.<br />
Dr Fagan: To be able to sit in the clinic with a patient and to have<br />
on a piece of paper an assessment about what they’re most likely<br />
to respond to is a powerful thought. It might limit the “wait and<br />
see” period after starting a treatment to see if it has any effect. It<br />
also might limit the possibility of losing ground.<br />
“My current work is<br />
looking at gene expression<br />
profiling and<br />
peripheral bloodcells<br />
and people with PH.<br />
My hypothesis is that<br />
we can gain information about PH by<br />
looking at immune-related cells from<br />
the blood. Part of the difficulty has<br />
always been that the lung tissue,<br />
where PH is manifest, is not readily<br />
accessible in our patients. So we<br />
need to find surrogate tissue to<br />
examine. We’re looking at the blood<br />
cells as a surrogate marker of the<br />
disease and have had some success<br />
using this approach.” –Dr Bull<br />
Dr McMurtry: <strong>No</strong>t only was Dr Weinshilboum’s talk on pharmacogenomics<br />
up to date and educational; it was also very entertaining.<br />
He is a personable and skillful lecturer, and anyone who<br />
gets the chance to hear him speak should definitely do so.<br />
Dr Fagan: I also thought Dale Able’s lecture on metabolic determinants<br />
of a cardiac myocyte performance was really intriguing.<br />
His work has illuminated the mechanisms of cardiac dysfunction<br />
associated with the metabolic syndrome. I was really impressed<br />
with his ability to make the incredible complex metabolic signaling<br />
pathways approachable to clinicians and to people who are<br />
nonmetabolic researchers. It really reminded me that our patients<br />
have comorbidities that they’re bringing to the table as well. So it’s<br />
not just what their genes are, it’s not just whether they have scleroderma<br />
or whether they’ve got congenital heart disease, but they<br />
bring all sorts of other things to the table that can directly impact<br />
their overall heath and PH treatment. Obviously one of those are<br />
people who have metabolic syndrome related<br />
to obesity. There are several articles that are<br />
beginning to look more carefully at the effects<br />
of metabolism on cardiac performance.<br />
It certainly spurred me to think about RV<br />
performance in a different way.<br />
Dr Minai: Several studies have established<br />
a clear link between the metabolic syndrome<br />
and cardiovascular disease. Despite recent<br />
studies, this link remains tantalizingly close<br />
but as yet unproven in PH. Studies looking<br />
at the association between PH and obesity<br />
and sleep apnea have reported mixed results.<br />
This is an area that requires further<br />
study.<br />
Dr Bull: We tend to hone in on the pulmonary<br />
vascular bed and the RV response to<br />
pressure overload but our patients live in the<br />
real world and have all the diseases of the real world and how<br />
these things interact in a more sophisticated manner is important<br />
to consider. So I thought that was a great lecture as well.<br />
Dr Fagan: The meeting gave us a chance to also look at clinical<br />
and translational research opportunities. I know that you, Todd,<br />
participated in some research at these scientific sessions and I<br />
was hoping you could tell us how you did it, some of the difficulties<br />
in doing research away from your own primary site and what<br />
you thought about how the meeting contributes to your research.<br />
Dr Bull: This is actually my second time doing research at the<br />
<strong>PHA</strong> conference. In Minneapolis I was working on the same project.<br />
It is only possible through the generosity of <strong>PHA</strong> and most importantly,<br />
the patients who attend the <strong>PHA</strong> meeting. My current<br />
work is looking at gene expression profiling and peripheral blood<br />
cells and people with PH. My hypothesis is that we can gain information<br />
about PH by looking at immune-related cells from the<br />
blood. Part of the difficulty has always been that the lung tissue,<br />
where PH is manifest, is not readily accessible in our patients.<br />
So we need to find surrogate tissue to examine. We’re looking at<br />
the blood cells as a surrogate marker of the disease and have had<br />
some success using this approach. This success has been in large<br />
part due to the <strong>PHA</strong> conference research. Greg Elliot really started<br />
354 Advances in Pulmonary Hypertension
performing research at the <strong>PHA</strong> conference at the very first meeting<br />
at Stone Mountain in Georgia. He realized then that in this rare<br />
disease a meeting where patients from all over the world come<br />
together would be a great opportunity to learn more about the disease<br />
and hopefully help improve how we diagnose and treat PH.<br />
In a one-day period at the <strong>PHA</strong> meeting, I can get a year’s worth<br />
of samples; it would take us that long to collect the same amount<br />
in our clinic and we have a pretty busy clinic. For my work, we obtain<br />
a blood sample, isolate the peripheral blood mononuclear cell<br />
component, and then look at gene expression. One factor is that<br />
after we draw the blood, we need to arrange to have a laboratory<br />
nearby available to us.<br />
Dr Fagan: How do you do that?<br />
Dr Bull: Through networking. At the <strong>University</strong> of Minnesota I got<br />
in touch with the chief of pulmonary who<br />
then put me in contact with a principal investigator<br />
in a lab that had the materials and<br />
equipment that I needed. We had to get IRB<br />
approval for these studies at the <strong>University</strong><br />
of Minnesota. We did the same thing in Houston.<br />
As soon as we were done drawing blood<br />
at the conference we rushed it over to the university<br />
where we pro-cessed samples, finishing<br />
at about 2 o’clock in the morning. It was<br />
an onerous process, but we got great samples<br />
that we hope will translate into great data.<br />
The next phase is to collect the clinical information<br />
from the study subjects.<br />
The most important part of this is really<br />
the patients’ participation. The patients have really been fantastic;<br />
they’ve lined up at lunchtime, in between their sessions, and<br />
are willing to donate blood. It is an amazing thing to see and we<br />
are very appreciative of their help.<br />
I think that at this most recent meeting there were at least 6<br />
to 7 groups doing research. There almost wasn’t enough room for<br />
us all in the research room. I think when we do this again we’ll<br />
have to get more space. It’s been working remarkably well and<br />
there’s potential to make it even better. Greg Elliot has been the<br />
driving force behind this so we owe him a lot.<br />
Dr Fagan: The focus of the <strong>PHA</strong> in terms of its advocacy for patients<br />
includes research. One great thing that I have noticed is<br />
that more and more non-clinician researchers are coming to the<br />
meeting, especially the Scientific Sessions. Ivan, you gave an excellent<br />
review of the current animal models of PH that are used<br />
in research. You are also a great example of non-clinicians who attend<br />
this meeting, many of whom are seeing patients with PH for<br />
the first time. Ivan, perhaps you could share your thoughts about<br />
the conference as a PhD scientist who’s done PH research for 40<br />
years with limited patient contact.<br />
Dr McMurtry: Attending and participating in the <strong>PHA</strong> meeting was<br />
an incredible experience for me. I’ve presented results of my research<br />
on animal models of PH at numerous scientific meetings<br />
over the past 40 years. This was the first time I had PH patients,<br />
and their family members, personally thank me for my contributions<br />
to their treatment. I actually got a sense of what it must be<br />
“Attending and participating<br />
in the <strong>PHA</strong><br />
meeting was an incredible<br />
experience<br />
for me. I’ve presented<br />
results of my research<br />
on animal models of PH at numerous<br />
scientific meetings over the past 40<br />
years. This was the first time I had<br />
PH patients, and their family members,<br />
personally thank me for my contributions<br />
to their treatment.”–Dr McMurtry<br />
like to be a rock star, since I had patients giving me hugs and requesting<br />
to have their pictures taken with me! It was a heartwarming<br />
and motivating experience, and I would recommend to<br />
any PhD scientist working in the field of PH to attend the meeting<br />
and experience first hand the appreciation and hope of PH<br />
patients.<br />
Dr Fagan: I was very fortunate to see some of the interactions that<br />
you had with the patients, Ivan. They really did acknowledge and<br />
appreciate your work. It was as meaningful to them as it was for<br />
you. It highlights that we really need to encourage non-clinician<br />
scientists to come to this conference even more. As a clinician I<br />
get a lot of appreciation from my patients all the time. I think people<br />
who are not in the clinic but are in the research lab don’t get<br />
to see the end result from the work they’re doing and how much<br />
their efforts have impacted on the lives of patients.<br />
Dr Minai: That is part of <strong>PHA</strong>’s strength that<br />
it brings together clinicians and researchers,<br />
physicians and patients, physicians from various<br />
medical fields including pulmonology,<br />
cardiology, and rheumatology for the sole<br />
purpose of improving care and outcomes in<br />
patients with PH. Since this is the largest<br />
PH conference in the world and draws physicians<br />
and patients from around the globe,<br />
this forum provides a unique opportunity for<br />
research that would not be possible at any<br />
single center. The <strong>PHA</strong> is committed to patient<br />
advocacy and research and therapeutic<br />
advancement in the field of PH and this<br />
conference is a true reflection of that commitment on several levels.<br />
Dr Bull: I think that’s a great point. I always leave the <strong>PHA</strong> meeting<br />
inspired. When you come to the <strong>PHA</strong> and you see the determination<br />
and energy, you’re reminded about why it is you do what<br />
you do, why you’ve selected this as your career. More people<br />
should have this experience<br />
Dr Fagan: I think many of us come to the <strong>PHA</strong> because we already<br />
have not just a professional but a personal commitment to the patients<br />
of the organization. We really need to get to our colleagues<br />
and say, yes this is a different meeting than you’ll ever go to, but<br />
it is very worthwhile. Here’s the opportunity to see some very high<br />
quality state-of-the-art science but these other days you have the<br />
chance to interact and see what your work has done for these patients.<br />
I think it’s important to encourage people to come and see<br />
that.<br />
Dr Fagan: I would like to thank you all for your participation in this<br />
discussion. It is remarkable to look back and see how far the PH<br />
community has come and the Scientific Sessions and International<br />
Conference are just a small part of that. I know that the<br />
Scientific Sessions will remain an important part of the meeting<br />
and am delighted that Dr. Ivan Robbins from Vanderbilt <strong>University</strong><br />
will be taking the lead role in the Scientific Sessions for 2010.<br />
Hopefully we can encourage new participants in the conference in<br />
the future. ■<br />
Advances in Pulmonary Hypertension 355
In the treatment of pulmonary arterial hypertension (PAH)<br />
(WHO Group 1, Class II or III symptoms)<br />
Living with PAH<br />
can be complicated...<br />
Choosing a therapy<br />
should not be.<br />
Please see below for important safety information, including<br />
boxed WARNINGS on the possible risk of liver injury and the<br />
risk of serious birth defects.<br />
INDICATION: LETAIRIS is an endothelin receptor antagonist indicated for<br />
the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1)<br />
in patients with WHO Class II or III symptoms to improve exercise capacity<br />
and delay clinical worsening.<br />
Clinical worsening is defined as the first occurrence of death, lung<br />
transplantation, hospitalization for PAH, atrial septostomy, study<br />
withdrawal due to the addition of other PAH therapeutic agents, or study<br />
withdrawal due to early escape. 1<br />
Early escape criteria were two or more of the following after a<br />
minimum treatment period of 4 weeks: ≥20% decrease in 6-minute walk<br />
distance; worsening WHO functional class; worsening right ventricular<br />
failure; rapidly progressing cardiac, hepatic, or renal failure; and refractory<br />
systolic hypotension 5× ULN or if elevations are accompanied by bilirubin >2× ULN<br />
or by signs or symptoms of liver dysfunction<br />
• May cause fetal harm if taken during pregnancy<br />
• Must exclude pregnancy before the start of treatment<br />
• Prevent pregnancy thereafter by the use of two reliable methods<br />
of contraception<br />
Important safety information regarding hepatotoxicity<br />
LETAIRIS is not recommended in patients with elevated aminotransferases<br />
(>3× ULN) at baseline because monitoring liver injury may be more difficult.<br />
If aminotransferase elevations are accompanied by clinical symptoms of<br />
liver injury (such as anorexia, nausea, vomiting, fever, malaise, fatigue, right<br />
upper quadrant abdominal discomfort, itching, or jaundice) or increases in<br />
bilirubin >2× ULN, LETAIRIS treatment should be stopped. There is no<br />
experience with the reintroduction of LETAIRIS in these circumstances.<br />
Contraindication<br />
• Do not administer LETAIRIS to a pregnant woman because it can cause<br />
fetal harm<br />
Warnings and precautions<br />
• Decreases in hemoglobin have been observed within the first few weeks<br />
of treatment with LETAIRIS; measure hemoglobin prior to initiation, at<br />
1 month, and periodically thereafter<br />
• Mild to moderate peripheral edema. Peripheral edema occurred more<br />
frequently in elderly patients (age ≥65 years) receiving LETAIRIS (29%;<br />
16/56) compared to placebo (4%; 1/28)<br />
• Peripheral edema is a known class effect of endothelin receptor<br />
antagonists. In addition, there have been postmarketing reports of fluid<br />
retention occurring within weeks after starting LETAIRIS which required<br />
intervention with a diuretic, fluid management, or, in some cases,<br />
hospitalization for decompensating heart failure<br />
Drug interactions<br />
• Use caution when LETAIRIS is coadministered with cyclosporine A<br />
• Use caution when LETAIRIS is coadministered with strong CYP3A<br />
inhibitors (e.g., ketoconazole) or CYP2C19 inhibitors (e.g., omeprazole)<br />
• Use caution when LETAIRIS is coadministered with inducers of P-gp,<br />
CYPs, and UGTs
Letairis® (ambrisentan) is indicated for the treatment of PAH (WHO Group 1, Class II or III symptoms)<br />
LETAIRIS offers<br />
Simple dosing<br />
One pill, once a day 1<br />
Two therapeutically effective doses 1<br />
• Available in 5 mg and 10 mg tablets<br />
• Initiate treatment at 5 mg once daily, and consider increasing the dose to 10 mg if 5 mg is tolerated<br />
Designed for everyday.<br />
Reliable improvements<br />
Up to +59 m placebo-adjusted mean change from baseline in 6MWD * at 12 weeks with LETAIRIS †1<br />
• LETAIRIS was studied in two 12-week, randomized, double-blind, placebo-controlled, multicenter studies (ARIES-1, N=201, and ARIES-2, N=192);<br />
6MWD was the primary endpoint 1<br />
—ARIES-1: +51 m (10 mg, p3% incidence in the combined LETAIRIS treatment<br />
group and more frequent than in the placebo group, with a difference of ≥1% between the<br />
LETAIRIS and placebo groups.<br />
* 6MWD=6-minute walk distance; baseline mean 6MWD was 341 ± 76 m in ARIES-1 and<br />
348 ± 84 m in ARIES-2. 2<br />
†<br />
Improvements in exercise capacity were greater for younger patients than for elderly patients<br />
(≥65 years), and greater for patients with idiopathic PAH (IPAH) than for those with associated<br />
PAH (APAH). Results of such subgroup analyses must be interpreted with caution.<br />
References: 1. LETAIRIS [Prescribing Information]. Foster City, Calif: Gilead Sciences, Inc;<br />
October 2008. 2. Data on file. Gilead Sciences, Inc.<br />
© 2008 Gilead Sciences, Inc. All rights reserved.<br />
ABS0130 December 2008<br />
LETAIRIS is a registered trademark and Gilead and the<br />
Gilead logo are trademarks of Gilead Sciences, Inc.
LETAIRIS® (ambrisentan) 5 mg and 10 mg Tablets<br />
Brief summary of full prescribing information. See full prescribing information. Rx only.<br />
WARNING: POTENTIAL LIVER INJURY<br />
LETAIRIS (ambrisentan) can cause elevation of liver aminotransferases (ALT and AST)<br />
to at least 3 times the upper limit of normal (ULN). LETAIRIS treatment was associated<br />
with aminotransferase elevations >3× ULN in 0.8% of patients in 12-week trials and<br />
2.8% of patients including long-term open-label trials out to one year. One case of<br />
aminotransferase elevations >3× ULN has been accompanied by bilirubin elevations<br />
>2× ULN. Because these changes are a marker for potentially serious liver injury,<br />
serum aminotransferase levels (and bilirubin if aminotransferase levels are elevated)<br />
must be measured prior to initiation of treatment and then monthly. In the postmarketing<br />
period with another endothelin receptor antagonist (ERA), bosentan, rare<br />
cases of unexplained hepatic cirrhosis were reported after prolonged (>12 months)<br />
therapy. In at least one case with bosentan, a late presentation (after >20 months of<br />
treatment) included pronounced elevations in aminotransferases and bilirubin levels<br />
accompanied by non-specific symptoms, all of which resolved slowly over time after<br />
discontinuation of the suspect drug. This case reinforces the importance of strict<br />
adherence to the monthly monitoring schedule for the duration of treatment.<br />
Elevations in aminotransferases require close attention. LETAIRIS should generally<br />
be avoided in patients with elevated aminotransferases (>3× ULN) at baseline<br />
because monitoring liver injury may be more difficult. If liver aminotransferase<br />
elevations are accompanied by clinical symptoms of liver injury (such as nausea,<br />
vomiting, fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases<br />
in bilirubin >2× ULN, treatment should be stopped. There is no experience with the<br />
re-introduction of LETAIRIS in these circumstances.<br />
CONTRAINDICATION: PREGNANCY<br />
LETAIRIS is very likely to produce serious birth defects if used by pregnant women, as<br />
this effect has been seen consistently when it is administered to animals [see<br />
Contraindications (4.1)]. Pregnancy must therefore be excluded before the initiation<br />
of treatment with LETAIRIS and prevented thereafter by the use of at least two<br />
reliable methods of contraception unless the patient has had a tubal sterilization or<br />
Copper T 380A IUD or LNg 20 IUD inserted, in which case no other contraception is<br />
needed. Obtain monthly pregnancy tests. Because of the risks of liver injury and birth<br />
defects, LETAIRIS is available only through a special restricted distribution program<br />
called the LETAIRIS Education and Access Program (LEAP), by calling 1-866-664-LEAP<br />
(5327). Only prescribers and pharmacies registered with LEAP may prescribe and<br />
distribute LETAIRIS. In addition, LETAIRIS may be dispensed only to patients who are<br />
enrolled in and meet all conditions of LEAP [see WARNINGS, Prescribing and Distribution<br />
Program for LETAIRIS].<br />
INDICATIONS AND USAGE: LETAIRIS is indicated for the treatment of pulmonar y ar terial hyper tension<br />
(WHO Group 1) in patients with WHO class II or III symptoms to improve exercise capacity and delay<br />
clinical worsening.<br />
DOSAGE AND ADMINISTRATION: Adult Dosage: Initiate treatment at 5 mg once daily with or<br />
without food, and consider increasing the dose to 10 mg once daily if 5 mg is tolerated.Tablets may be<br />
administered with or without food. Tablets should not be split, crushed, or chewed. Doses higher<br />
than 10 mg once daily have not been studied in patients with pulmonary arterial hypertension (PAH).<br />
Liver function tests should be measured prior to initiation and during treatment with LETAIRIS [see<br />
Warnings and Precautions (5.1)]. Women of Childbearing Potential: Pregnancy tests should be<br />
obtained monthly in women of childbearing potential taking LETAIRIS [see Contraindications (4.1)].<br />
Pre-existing Hepatic Impairment: LETAIRIS is not recommended in patients with moderate or<br />
severe hepatic impairment [see Special Populations (8.7)].<br />
CONTRAINDICATIONS: Pregnancy Category X: Teratogenicity is a class effect of endothelin<br />
receptor antagonists. There are no data on the use of LETAIRIS in pregnant women. LETAIRIS is<br />
contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or<br />
if the patient becomes pregnant while taking this drug, the patient should be apprised of the<br />
potential hazard to a fetus.<br />
WARNINGS AND PRECAUTIONS: Potential Liver Injury (see BOXED WARNING): Treatment with<br />
endothelin receptor antagonists has been associated with dose-dependent liver injury manifested<br />
primarily by elevation of serum aminotransferases (ALT or AST), but sometimes accompanied by<br />
abnormal liver function (elevated bilirubin). The combination of aminotransferases greater than<br />
3-times the upper limit of normal (>3× ULN) and total bilirubin >2× ULN is a marker for potentially<br />
serious hepatic injury. Liver function tests were closely monitored in all clinical studies with LETAIRIS.<br />
For all LETAIRIS-treated patients (N=483), the 12-week incidence of aminotransferases >3× ULN<br />
was 0.8% and >8× ULN was 0.2%. Liver chemistries must be measured prior to initiation of LETAIRIS<br />
and at least every month thereafter. If there are aminotransferase elevations >3× ULN and ≤5×<br />
ULN, they should be re-measured. If the confirmed level is >3× ULN and ≤5× ULN, reduce the daily<br />
dose or interrupt treatment and continue to monitor every two weeks until the levels are 5× ULN and ≤8× ULN, LETAIRIS should be discontinued<br />
and monitoring should continue until the levels are 8× ULN, treatment should be stopped and re-initiation should not be considered. If aminotransferase<br />
elevations are accompanied by clinical symptoms of liver injury (such as anorexia, nausea, vomiting,<br />
fever, malaise, fatigue, right upper quadrant abdominal discomfort, itching, or jaundice) or increases<br />
in bilirubin >2× ULN, LETAIRIS treatment should be stopped. Hematological Changes: Decreases<br />
in hemoglobin concentration and hematocrit have followed administration of other endothelin<br />
receptor antagonists and were observed in clinical studies with LETAIRIS. These decreases were<br />
observed within the first few weeks of treatment with LETAIRIS, and stabilized thereafter. The mean<br />
decrease in hemoglobin from baseline to end of treatment for those patients receiving LETAIRIS in<br />
the 12-week placebo-controlled studies was 0.8 g/dL. Marked decreases in hemoglobin (>15%<br />
decrease from baseline resulting in a value below the lower limit of normal) were observed in 7% of<br />
all patients receiving LETAIRIS (and 10% of patients receiving 10 mg) compared to 4% of patients<br />
receiving placebo. The cause of the decrease in hemoglobin is unknown, but it does not appear to<br />
result from hemorrhage or hemolysis. Hemoglobin must be measured prior to initiation of LETAIRIS<br />
and should be measured at one month and periodically thereafter. If a clinically significant decrease<br />
in hemoglobin is observed and other causes have been excluded, discontinuation of treatment<br />
should be considered. Peripheral Edema: Peripheral edema is a known class effect of endothelin<br />
receptor antagonists. In addition, there have been post-marketing reports of fluid retention occurring<br />
within weeks after starting LETAIRIS which required intervention with a diuretic, fluid management,<br />
or, in some cases, hospitalization for decompensating heart failure. Co-administration of LETAIRIS<br />
and Cyclosporine A: Cyclosporine is a strong inhibitor of P-glycoprotein (P-gp), Organic Anion<br />
Transport Protein (OATP), and CYP3A4. In vitro data indicate ambrisentan is a substrate of P-gp, OATP<br />
and CYP3A. Therefore, use caution when LETAIRIS is co-administered with cyclosporine A because<br />
cyclosporine A may cause increased exposure to LETAIRIS [see Drug Interactions (7)]. Coadministration<br />
of LETAIRIS and Strong CYP3A and 2C19 Inhibitors: Use caution when LETAIRIS<br />
is co-administered with strong CYP3A-inhibitors (e.g., ketoconazole) and CYP2C19-inhibitors (e.g.,<br />
omeprazole) [see Drug Interactions (7)]. Prescribing and Distribution Program for LETAIRIS:<br />
Because of the risks of liver injury and birth defects, LETAIRIS is available only through a special<br />
restricted distribution program called the LETAIRIS Education and Access Program (LEAP). Only<br />
prescribers and pharmacies registered with LEAP may prescribe and distribute LETAIRIS. In addition,<br />
LETAIRIS® (ambrisentan) may be dispensed only to patients who are enrolled in and meet all<br />
conditions of LEAP. To enroll or receive more information visit www.letairis.com or call 1-866-664-LEAP<br />
(5327).<br />
ADVERSE REACTIONS: Clinical Trials Experience: Safety data for LETAIRIS were obtained from<br />
two 12-week, placebo-controlled studies in patients with PAH (ARIES-1 and ARIES-2) and four<br />
nonplacebo-controlled studies in 483 patients with PAH who were treated with doses of 1, 2.5, 5, or<br />
10 mg once daily. The exposure to LETAIRIS in these studies ranged from 1 day to 4 years (N=418 for<br />
at least 6 months and N=343 for at least 1 year). In ARIES-1 and ARIES-2, a total of 261 patients<br />
received LETAIRIS at doses of 2.5, 5, or 10 mg once daily and 132 patients received placebo. The<br />
adverse events that occurred in >3% of the patients receiving LETAIRIS and were more frequent on<br />
LETAIRIS than placebo are shown in Table 1.<br />
Table 1 Adverse Events in >3% of PAH Patients Receiving<br />
LETAIRIS and More Frequent than Placebo<br />
Placebo (N=132)<br />
LETAIRIS (N=261)<br />
Adverse event n (%) n (%) Placebo-adjusted (%)<br />
Peripheral edema 14 (11) 45 (17) 6<br />
Nasal congestion 2 (2) 15 (6) 4<br />
Sinusitis 0 (0) 8 (3) 3<br />
Flushing 1 (1) 10 (4) 3<br />
Palpitations 3 (2) 12 (5) 3<br />
Nasopharyngitis 1 (1) 9 (3) 2<br />
Abdominal pain 1 (1) 8 (3) 2<br />
Constipation 2 (2) 10 (4) 2<br />
Dyspnea 4 (3) 11 (4) 1<br />
Headache 18 (14) 38 (15) 1<br />
<strong>No</strong>te: This table includes all adverse events >3% incidence in the combined LETAIRIS treatment group and more<br />
frequent than in the placebo group, with a difference of ≥1% between the LETAIRIS and placebo groups.<br />
Most adverse drug reactions were mild to moderate and only nasal congestion was dose-dependent.<br />
Fewer patients receiving LETAIRIS had adverse events related to liver function tests compared to<br />
placebo. Peripheral edema was similar in younger patients (
START WITH CONFIDENCE<br />
REVATIO: for patients with PAH as early as class II<br />
• REVATIO is indicated for the treatment of<br />
pulmonary arterial hypertension (WHO<br />
Group I) to improve exercise ability<br />
– WHO Group I<br />
• A first-line treatment for class II and<br />
class III 1<br />
– Updated American College of Chest Physicians<br />
evidence-based clinical practice guidelines<br />
• The lowest-priced oral PAH therapy*<br />
– REVATIO 20 mg tid<br />
*Based on wholesale acquisition cost: First DataBank Inc., 2008. Actual<br />
pharmacy or out-of-pocket costs may vary. Price comparisons do<br />
not imply comparable efficacy and safety. The pivotal trial for REVATIO<br />
included patients who were predominantly functional classes II and III,<br />
and the pivotal trial for Tracleer ® included patients who were<br />
predominantly functional class III.<br />
REVATIO is indicated for the treatment of pulmonary arterial hypertension (WHO Group I) to improve exercise ability. The efficacy of REVATIO has not been<br />
evaluated in patients currently on bosentan therapy.<br />
The use of REVATIO and organic nitrates in any form, at any time, is contraindicated.<br />
Co-administration of REVATIO with potent CYP3A4 inhibitors (eg, ketoconazole, itraconazole, and ritonavir) is not recommended as serum<br />
concentrations of sildenafil substantially increase. Co-administration of REVATIO with CYP3A4 inducers, including bosentan; and more potent inducers<br />
such as barbiturates, carbamazepine, phenytoin, efavirenz, nevirapine, rifampin, and rifabutin, may alter plasma levels of either or both<br />
medications. Dosage adjustment may be necessary.<br />
Before starting REVATIO, physicians should carefully consider whether their patients with underlying conditions could be adversely affected by the mild<br />
and transient vasodilatory effects of REVATIO on blood pressure. Pulmonary vasodilators may significantly worsen the cardiovascular status of patients<br />
with pulmonary veno-occlusive disease (PVOD) and administration of REVATIO to these patients is not recommended. Should signs of<br />
pulmonary edema occur when sildenafil is administered, the possibility of associated PVOD should be considered.<br />
The most common side effects of REVATIO (placebo-subtracted) were epistaxis (8%), headache (7%), dyspepsia (6%), flushing (6%), and insomnia (6%).<br />
Adverse events were generally transient and mild to moderate.<br />
Caution is advised when PDE5 inhibitors, such as REVATIO, are administered with -blockers as both are vasodilators with blood pressure lowering effects.<br />
REVATIO should be used with caution in patients with anatomical deformation of the penis or patients who have conditions which may predispose them to priapism.<br />
In PAH patients, the concomitant use of vitamin K antagonists and REVATIO resulted in a greater incidence of reports of bleeding (primarily epistaxis)<br />
versus placebo. The incidence of epistaxis was higher in patients with PAH secondary to CTD (sildenafil 13%, placebo 0%) than in PPH patients<br />
(sildenafil 3%, placebo 2%).<br />
<strong>No</strong>n-arteritic anterior ischemic optic neuropathy (NAION) has been reported rarely post-marketing<br />
in temporal association with the use of PDE5 inhibitors for the treatment of erectile dysfunction,<br />
including sildenafil. It is not possible to determine if these events are related to PDE5<br />
inhibitors or to other factors. Physicians should advise patients to seek immediate medical<br />
attention in the event of sudden loss of vision while taking PDE5 inhibitors, including REVATIO.<br />
Sudden decrease or loss of hearing has been reported in temporal association with the intake<br />
of PDE5 inhibitors, including REVATIO. It is not possible to determine whether these events are<br />
related directly to the use of PDE5 inhibitors or to other factors. Physicians should advise<br />
patients to seek prompt medical attention in the event of sudden decrease or loss of<br />
hearing while taking PDE5 inhibitors, including REVATIO.<br />
Tracleer (bosentan) is a registered trademark of Actelion Pharmaceuticals.<br />
Please see brief summary of prescribing information on adjacent page.<br />
REVATIO contains sildenafil citrate, the<br />
same active ingredient found in Viagra ®<br />
www.pfizerpro.com
Reference: 1. Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917-1928.<br />
Brief summary of prescribing information<br />
INDICATIONS AND USAGE<br />
REVATIO is indicated for the treatment of pulmonary arterial hypertension (WHO Group I) to improve exercise ability.<br />
The efficacy of REVATIO has not been evaluated in patients currently on bosentan therapy.<br />
CONTRAINDICATIONS<br />
Consistent with its known effects on the nitric oxide/cGMP pathway (see CLINICAL <strong>PHA</strong>RMACOLOGY), sildenafil was shown<br />
to potentiate the hypotensive effects of nitrates, and its administration to patients who are using organic nitrates, either<br />
regularly and/or intermittently, in any form is therefore contraindicated.<br />
REVATIO is contraindicated in patients with a known hypersensitivity to any component of the tablet.<br />
WARNINGS<br />
The concomitant administration of the protease inhibitor ritonavir (a highly potent CYP3A4 inhibitor) substantially increases<br />
serum concentrations of sildenafil, therefore co-administration with REVATIO is not recommended (see Drug Interactions and<br />
DOSAGE AND ADMINISTRATION).<br />
REVATIO has vasodilator properties, resulting in mild and transient decreases in blood pressure (see<br />
PRECAUTIONS). Prior to prescribing REVATIO, physicians should carefully consider whether their patients with<br />
certain underlying conditions could be adversely affected by such vasodilatory effects, for example patients with resting<br />
hypotension (BP 170/110);<br />
• Patients with retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases);<br />
• Patients currently on bosentan therapy.<br />
PRECAUTIONS<br />
General<br />
Before prescribing REVATIO, it is important to note the following:<br />
• Caution is advised when phosphodiesterase type 5 (PDE5) inhibitors are co-administered with alpha-blockers. PDE5<br />
inhibitors, including sildenafil, and alpha-adrenergic blocking agents are both vasodilators with blood pressure lowering<br />
effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some<br />
patients, concomitant use of these two drug classes can lower blood pressure significantly, leading to symptomatic<br />
hypotension. In the sildenafil interaction studies with alpha-blockers (see Drug Interactions), cases of symptomatic<br />
hypotension consisting of dizziness and lightheadedness were reported. <strong>No</strong> cases of syncope or fainting were reported<br />
during these interaction studies. Consideration should be given to the fact that safety of combined use of PDE5 inhibitors<br />
and alpha-blockers may be affected by other variables, including intravascular volume depletion and concomitant use of<br />
anti-hypertensive drugs.<br />
• REVATIO should be used with caution in patients with anatomical deformation of the penis (such as angulation,<br />
cavernosal fibrosis or Peyronie’s disease) or in patients who have conditions, which may predispose them to priapism (such<br />
as sickle cell anemia, multiple myeloma or leukemia). In the event of an erection that persists longer than 4 hours, the<br />
patient should seek immediate medical assistance. If priapism (painful erections greater than 6 hours in duration) is not<br />
treated immediately, penile tissue damage and permanent loss of potency could result.<br />
• In humans, sildenafil has no effect on bleeding time when taken alone or with aspirin. In vitro studies with human platelets<br />
indicate that sildenafil potentiates the anti-aggregatory effect of sodium nitroprusside (a nitric oxide donor). The<br />
combination of heparin and sildenafil had an additive effect on bleeding time in the anesthetized rabbit, but this<br />
interaction has not been studied in humans.<br />
• The incidence of epistaxis was higher in patients with PAH secondary to CTD (sildenafil 13%, placebo 0%) than in PPH<br />
patients (sildenafil 3%, placebo 2%). The incidence of epistaxis was also higher in sildenafil-treated patients with<br />
concomitant oral vitamin K antagonist (9% versus 2% in those not treated with concomitant vitamin K antagonist).<br />
• The safety of REVATIO is unknown in patients with bleeding disorders and patients with active peptic ulceration.<br />
Information for Patients<br />
Physicians should discuss with patients the contraindication of REVATIO with regular and/or intermittent use of<br />
organic nitrates.<br />
Sildenafil is also marketed as VIAGRA ® for male erectile dysfunction.<br />
Physicians should advise patients to seek immediate medical attention in the event of a sudden loss of vision in one or both<br />
eyes while taking all PDE5 inhibitors, including REVATIO. Such an event may be a sign of non-arteritic anterior ischemic optic<br />
neuropathy (NAION), a cause of decreased vision including permanent loss of vision, that has been reported rarely postmarketing<br />
in temporal association with the use of all PDE5 inhibitors when used in the treatment of male erectile<br />
dysfunction. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other<br />
factors. Physicians should also discuss with patients the increased risk of NAION in individuals who have already experienced<br />
NAION in one eye, including whether such individuals could be adversely affected by use of vasodilators, such as PDE5<br />
inhibitors (see ADVERSE REACTIONS).<br />
Physicians should advise patients to seek prompt medical attention in the event of sudden decrease or loss of hearing while<br />
taking all PDE5 inhibitors, including REVATIO. These events, which may be accompanied by tinnitus and dizziness, have been<br />
reported in temporal association to the intake of PDE5 inhibitors, including REVATIO. It is not possible to determine whether<br />
these events are related directly to the use of PDE5 inhibitors or to other factors (see ADVERSE REACTIONS, Clinical Trials<br />
and Post-Marketing Experience).<br />
Drug Interactions<br />
In PAH patients, the concomitant use of vitamin K antagonists and sildenafil resulted in a greater incidence of reports of<br />
bleeding (primarily epistaxis) versus placebo.<br />
Effects of Other Drugs on REVATIO<br />
In vitro studies: Sildenafil metabolism is principally mediated by the CYP3A4 (major route) and CYP2C9 (minor route)<br />
cytochrome P450 isoforms. Therefore, inhibitors of these isoenzymes may reduce sildenafil clearance and inducers of these<br />
isoenzymes may increase sildenafil clearance.<br />
In vivo studies: Population pharmacokinetic analysis of clinical trial data indicated a reduction in sildenafil<br />
clearance and/or an increase of oral bioavailability when co-administered with CYP3A4 substrates and the<br />
combination of CYP3A4 substrates and beta-blockers. These were the only factors with a statistically significant impact on<br />
sildenafil pharmacokinetics.<br />
Population data from patients in clinical trials indicated a reduction in sildenafil clearance when it was<br />
co-administered with CYP3A4 inhibitors. Sildenafil exposure without concomitant medication is shown to be<br />
5-fold higher at a dose of 80 mg t.i.d. compared to its exposure at a dose of 20 mg t.i.d. This concentration range covers the<br />
same increased sildenafil exposure observed in specifically-designed drug interaction studies with CYP3A4 inhibitors (except<br />
for potent inhibitors such as ketoconazole, itraconazole, and ritonavir). Cimetidine (800 mg), a nonspecific CYP inhibitor,<br />
caused a 56% increase in plasma sildenafil concentrations when co-administered with sildenafil (50 mg) to healthy<br />
volunteers. When a single 100 mg dose of sildenafil was co-administered with erythromycin, a CYP3A4 inhibitor, at steady<br />
state (500 mg twice daily [b.i.d.] for 5 days), there was a 182% increase in sildenafil systemic exposure (AUC). In a study<br />
performed in healthy volunteers, co-administration of the HIV protease inhibitor saquinavir, a CYP3A4 inhibitor, at steady state<br />
(1200 mg t.i.d.) with sildenafil (100 mg single dose) resulted in a 140% increase in sildenafil C max and a 210% increase in<br />
sildenafil AUC. Stronger CYP3A4 inhibitors will have still greater effects on plasma levels of sildenafil<br />
(see DOSAGE AND ADMINISTRATION).<br />
In another study in healthy volunteers, co-administration with the HIV protease inhibitor ritonavir, a potent CYP3A4 inhibitor,<br />
at steady state (500 mg b.i.d.) with sildenafil (100 mg single dose) resulted in a 300% (4-fold) increase in sildenafil C max and<br />
a 1000% (11-fold) increase in sildenafil plasma AUC. At 24 hours, the plasma levels of sildenafil were still approximately<br />
200 ng/mL, compared to approximately 5 ng/mL when sildenafil was dosed alone. This is consistent with ritonavir's marked<br />
effects on a broad range of P450 substrates (see WARNINGS and DOSAGE AND ADMINISTRATION). Although the interaction<br />
between other protease inhibitors and REVATIO has not been studied, their concomitant use is expected to increase<br />
sildenafil levels.<br />
In a study of healthy male volunteers, co-administration of sildenafil at steady state (80 mg t.i.d.), with the endothelin<br />
receptor antagonist bosentan (a moderate inducer of CYP3A4, CYP2C9 and possibly of cytochrome P450 2C19) at steady state<br />
(125 mg b.i.d.) resulted in a 63% decrease of sildenafil AUC and a 55% decrease in sildenafil C max. The combination of both<br />
drugs did not lead to clinically significant changes in blood pressure (supine or standing). Concomitant administration of<br />
potent CYP3A4 inducers is expected to cause greater decreases in plasma levels of sildenafil.<br />
In drug-drug interaction studies, sildenafil (25 mg, 50 mg, or 100 mg) and the alpha-blocker doxazosin (4 mg or 8 mg) were<br />
administered simultaneously to patients with benign prostatic hyperplasia (BPH) stabilized on doxazosin therapy. In these<br />
study populations, mean additional reductions of supine systolic and diastolic blood pressure of 7/7 mmHg, 9/5 mmHg, and<br />
8/4 mmHg, respectively, were observed. Mean additional reductions of standing blood pressure of 6/6 mmHg, 11/4 mmHg,<br />
and 4/5 mmHg, respectively, were also observed. There were infrequent reports of patients who experienced symptomatic<br />
postural hypotension. These reports included dizziness and light-headedness, but not syncope (see PRECAUTIONS: General).<br />
Concomitant administration of oral contraceptives (ethinyl estradiol 30 µg and levonorgestrel 150 µg) did not affect the<br />
pharmacokinetics of sildenafil.<br />
Concomitant administration of a single 100 mg dose of sildenafil with 10 mg of atorvastatin did not alter the<br />
pharmacokinetics of either sildenafil or atorvastatin.<br />
Single doses of antacid (magnesium hydroxide/aluminum hydroxide) did not affect the bioavailability of sildenafil.<br />
Effects of REVATIO on Other Drugs<br />
In vitro studies: Sildenafil is a weak inhibitor of the cytochrome P450 isoforms 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4<br />
(IC50 >150 µM).<br />
In vivo studies: When sildenafil 100 mg oral was co-administered with amlodipine, 5 mg or 10 mg oral, to<br />
hypertensive patients, the mean additional reduction on supine blood pressure was 8 mmHg systolic and<br />
7 mmHg diastolic.<br />
<strong>No</strong> significant interactions were shown with tolbutamide (250 mg) or warfarin (40 mg), both of which are metabolized<br />
by CYP2C9.<br />
Sildenafil (50 mg) did not potentiate the increase in bleeding time caused by aspirin (150 mg).<br />
Sildenafil (50 mg) did not potentiate the hypotensive effect of alcohol in healthy volunteers with mean maximum blood<br />
alcohol levels of 0.08%.<br />
In healthy subjects, co-administration of 125 mg b.i.d. bosentan and 80 mg t.i.d. sildenafil resulted in a 63% decrease in<br />
AUC of sildenafil and a 50% increase in AUC of bosentan.<br />
In a study of healthy volunteers, sildenafil (100 mg) did not affect the steady-state pharmacokinetics of the HIV protease<br />
inhibitors saquinavir and ritonavir, both of which are CYP3A4 substrates.<br />
Sildenafil had no impact on the plasma levels of oral contraceptives (ethinyl estradiol 30 µg and levonorgestrel 150 µg).<br />
Carcinogenesis, Mutagenesis, Impairment of Fertility<br />
Sildenafil was not carcinogenic when administered to rats for up to 24 months at 60 mg/kg/day, a dose<br />
resulting in total systemic exposure (AUC) to unbound sildenafil and its major metabolite 33 and 37 times, for male and<br />
female rats, respectively, the human exposure at the Recommended Human Dose (RHD) of 20 mg t.i.d. Sildenafil was not<br />
carcinogenic when administered to male and female mice for up to 21 and 18 months, respectively, at doses up to a<br />
maximally tolerated level of 10 mg/kg/day, a dose equivalent to the RHD on a mg/m 2 basis.<br />
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human<br />
lymphocyte and in vitro mouse micronucleus assays to detect clastogenicity.<br />
There was no impairment of fertility in male or female rats given up to 60 mg sildenafil/kg/day, a dose<br />
producing a total systemic exposure (AUC) to unbound sildenafil and its major metabolite 19 and 38 times, for males and<br />
females, respectively, the human exposure at the RHD of 20 mg t.i.d.<br />
Pregnancy<br />
Pregnancy Category B. <strong>No</strong> evidence of teratogenicity, embryotoxicity or fetotoxicity was observed in pregnant rats or<br />
rabbits, dosed with 200 mg sildenafil/kg/day during organogenesis, a level that is, on a mg/m 2 basis,<br />
32- and 68-times, respectively, the RHD of 20 mg t.i.d. In a rat pre- and postnatal development study, the<br />
no-observed-adverse-effect dose was 30 mg/kg/day (equivalent to 5-times the RHD on a mg/m 2 basis). There are no<br />
adequate and well-controlled studies of sildenafil in pregnant women.<br />
Nursing Mothers<br />
It is not known if sildenafil citrate and/or metabolites are excreted in human breast milk. Since many drugs are excreted in<br />
human milk, caution should be used when REVATIO is administered to nursing women.<br />
Pediatric Use<br />
Safety and Effectiveness of sildenafil in pediatric pulmonary hypertension patients has not been established.<br />
Geriatric Use<br />
Healthy elderly volunteers (65 years or over) had a reduced clearance of sildenafil, but studies did not include sufficient<br />
numbers of subjects to determine whether they respond differently from younger subjects. Other reported clinical<br />
experience has not identified differences in response between the elderly and younger pulmonary arterial hypertension<br />
patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased<br />
hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.<br />
ADVERSE REACTIONS<br />
Clinical Trials<br />
Safety data were obtained from the pivotal study and an open-label extension study in 277 treated patients with pulmonary<br />
arterial hypertension. Doses up to 80 mg t.i.d. were studied.<br />
The overall frequency of discontinuation in REVATIO-treated patients at the recommended dose of 20 mg t.i.d. was low (3%)<br />
and the same as placebo (3%).<br />
In the pivotal placebo-controlled trial in pulmonary arterial hypertension, the adverse drug reactions that were reported by<br />
at least 3% of REVATIO patients treated at the recommended dosage (20 mg t.i.d.) and were more frequent in REVATIO<br />
patients than placebo patients, are shown in Table 1. Adverse events were generally transient and mild to moderate in<br />
nature.<br />
Table 1. Sildenafil Adverse Events in 3% of Patients and More Frequent Than Placebo<br />
ADVERSE EVENT %<br />
Epistaxis<br />
Headache<br />
Dyspepsia<br />
Flushing<br />
Insomnia<br />
Erythema<br />
Dyspnea exacerbated<br />
Rhinitis nos<br />
Diarrhea nos<br />
Myalgia<br />
Pyrexia<br />
Gastritis nos<br />
Sinusitis<br />
Paresthesia<br />
Placebo (n=70) Sildenafil 20 mg t.i.d. (n=69) Placebo Subtracted<br />
1<br />
9<br />
8<br />
39 46<br />
7<br />
741130643000 13<br />
6<br />
10 6<br />
7674976333 6<br />
5<br />
4<br />
4<br />
3<br />
3<br />
3<br />
3<br />
3<br />
3<br />
At doses higher than the recommended 20 mg t.i.d. there was a greater incidence of some adverse events including<br />
flushing, diarrhea, myalgia and visual disturbances. Visual disturbances were identified as mild and transient, and were<br />
predominately color-tinge to vision, but also increased sensitivity to light or blurred vision.<br />
In the pivotal study, the incidence of retinal hemorrhage at the recommended sildenafil 20 mg t.i.d. dose was 1.4% versus<br />
0% placebo and for all sildenafil doses studied was 1.9% versus 0% placebo. The incidence of eye hemorrhage at both the<br />
recommended dose and at all doses studied was 1.4% for sildenafil versus 1.4% for placebo. The patients experiencing<br />
these events had risk factors for hemorrhage including concurrent anticoagulant therapy.<br />
Post-Marketing Experience<br />
In post-marketing experience with sildenafil citrate at doses indicated for male erectile dysfunction, serious<br />
cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death,<br />
ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, pulmonary<br />
hemorrhage, and subarachnoid and intracerebral hemorrhages have been reported in temporal association with the use of<br />
the drug. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were<br />
reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of sildenafil<br />
without sexual activity. Others were reported to have occurred hours to days after use concurent with sexual activity. It is<br />
not possible to determine whether these events are related directly to sildenafil citrate, to sexual activity, to the patient’s<br />
underlying cardiovascular disease, or to a combination of these or other factors.<br />
When used to treat male-erectile dysfunction, non-arteritic anterior ischemic optic neuropathy (NAION), a cause of<br />
decreased vision including permanent loss of vision, has been reported rarely post-marketing in temporal association with<br />
the use of phosphodiesterase type 5 (PDE5) inhibitors, including sildenafil citrate. Most, but not all, of these patients had<br />
underlying anatomic or vascular risk factors for developing NAION, including but not necessarily limited to: low cup to disc<br />
ratio (“crowded disc”), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking. It is not<br />
possible to determine whether these events are related directly to the use of PDE5 inhibitors, to the patient’s underlying<br />
vascular risk factors or anatomical defects, to a combination of these factors, or to other factors (see PRECAUTIONS/<br />
Information for Patients).<br />
Cases of sudden decrease or loss of hearing have been reported post-marketing in temporal association with the use of<br />
PDE5 inhibitors, including REVATIO. In some of the cases, medical conditions and other factors were reported that may have<br />
also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible<br />
to determine whether these reported events are related directly to the use of REVATIO, to the patient’s underlying risk<br />
factors for hearing loss, a combination of these factors, or to other factors (see PRECAUTIONS, Information for Patients).<br />
OVERDOSAGE<br />
In studies with healthy volunteers of single doses up to 800 mg, adverse events were similar to those seen at lower doses<br />
but rates were increased.<br />
In cases of overdose, standard supportive measures should be adopted as required. Renal dialysis is not expected to<br />
accelerate clearance as sildenafil is highly bound to plasma proteins and it is not eliminated in the urine.<br />
October 2007<br />
RVU00055 ©2008 Pfizer Inc. All rights reserved. Printed in USA/August 2008<br />
U.S. Pharmaceutical
In pulmonary arterial hypertension (PAH)<br />
WHO Class III or IV<br />
1<br />
Tracleer goes beyond<br />
symptomatic relief<br />
*<br />
*<br />
†<br />
*BREATHE-1 Multicenter, randomized, double-blind,<br />
placebo-controlled study to assess the efficacy and safety<br />
of Tracleer (125 mg BID, 250 mg BID) in patients with WHO<br />
functional class III or IV PAH (N=213). All patients (n=144,<br />
Tracleer group; n=69, control group) participated in the first<br />
16 weeks. A subset of this population (n=35, Tracleer group;<br />
n=13, control group) continued for up to 28 weeks.<br />
Clinical worsening was defined as the combined endpoint<br />
of death, hospitalization for treatment related to PAH,<br />
discontinuation of therapy due to worsening PAH, or<br />
initiation of epoprostenol therapy. 2<br />
WHO functional class status 1,2 Placebo—Baseline: 94.2%<br />
Class III, 5.8% Class IV. Week 16: 0% Class I, 27.5% Class II,<br />
63.8% Class III, 8.7% Class IV. Tracleer—Baseline: 90.3%<br />
Class III, 9.7% Class IV. Week 16: 2.1% Class I, 36.1% Class<br />
II, 56.3% Class III, 5.6% Class IV.<br />
†Study 351 Randomized, double-blind, placebo-controlled<br />
study of Tracleer 125 mg BID in patients with WHO<br />
functional class III or IV PAH (N=32). 3<br />
RAP, right atrial pressure; CI, cardiac index; PVR, pulmonary<br />
vascular resistance; PAP, pulmonary arterial pressure<br />
Please visit www.TRACLEER.com to learn more<br />
about Tracleer and PAH.<br />
A Cornerstone of<br />
Oral Therapy<br />
Please see brief summary of prescribing information,<br />
including boxed warnings, on following page.<br />
Important safety information<br />
Liver<br />
and pregnancy warnings: Potential<br />
for<br />
serious liver injury (including,<br />
after<br />
prolonged treatment, rare cases of<br />
liver<br />
failure and unexplained hepatic cirrhosis in a setting of<br />
close monitoring)—Liver monitoring<br />
of all patients is essential prior to initiation n of treatment and monthly thereafter. e<br />
High potential for major birth defects—<br />
Pregnancy<br />
must<br />
be excluded and prevented<br />
by<br />
two forms<br />
of<br />
birth control;<br />
monthly<br />
pregnancy<br />
tests<br />
should be<br />
obtained.<br />
Because<br />
of<br />
these risks, Tracleer<br />
may<br />
only<br />
be prescribed through the Tracleer<br />
Access<br />
Program.<br />
Contraindicated icated for<br />
use with cyclosporine A and glyburide.
Use of TRACLEER ® requires attention to two significant concerns: 1) potential for serious liver injury, and 2) potential<br />
damage to a fetus.<br />
WARNING: Potential liver injury<br />
TRACLEER ® causes at least 3-fold (upper limit of normal; ULN) elevation of liver aminotransferases (ALT and AST) in<br />
about 11% of patients, accompanied by elevated bilirubin in a small number of cases. Because these changes are a<br />
marker for potential serious liver injury, serum aminotransferase levels must be measured prior to initiation of treatment<br />
and then monthly (see WARNINGS: Potential Liver Injury). In the post-marketing period, in the setting of<br />
close monitoring, rare cases of unexplained hepatic cirrhosis were reported after prolonged (>12 months) therapy<br />
with TRACLEER ® in patients with multiple co-morbidities and drug therapies. There have also been rare reports of liver<br />
failure. The contribution of TRACLEER ® in these cases could not be excluded.<br />
In at least one case the initial presentation (after > 20 months of treatment) included pronounced elevations in<br />
aminotransferases and bilirubin levels accompanied by non-specific symptoms, all of which resolved slowly over time<br />
after discontinuation of TRACLEER ® . This case reinforces the importance of strict adherence to the monthly monitoring<br />
schedule for the duration of treatment and the treatment algorithm, which includes stopping TRACLEER ® with a rise of<br />
aminotransferases accompanied by signs or symptoms of liver dysfunction.<br />
Elevations in aminotransferases require close attention. TRACLEER ® should generally be avoided in patients with<br />
elevated aminotransferases (> 3 x ULN) at baseline because monitoring liver injury may be more difficult. If liver<br />
aminotransferase elevations are accompanied by clinical symptoms of liver injury (such as nausea, vomiting,<br />
fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥ 2 x ULN, treatment should<br />
be stopped. There is no experience with the re-introduction of TRACLEER ® in these circumstances.<br />
CONTRAINDICATION: Pregnancy. TRACLEER ® (bosentan) is very likely to produce major birth defects if used by pregnant<br />
women, as this effect has been seen consistently when it is administered to animals (see CONTRAINDICATIONS).<br />
Therefore, pregnancy must be excluded before the start of treatment with TRACLEER ® and prevented thereafter by<br />
the use of a reliable method of contraception. Hormonal contraceptives, including oral, injectable, transdermal, and<br />
implantable contraceptives should not be used as the sole means of contraception because these may not be effective<br />
in patients receiving TRACLEER ® (see PRECAUTIONS: Drug Interactions). Therefore, effective contraception through<br />
additional forms of contraception must be practiced. Monthly pregnancy tests should be obtained.<br />
Because of potential liver injury and in an effort to make the chance of fetal exposure to TRACLEER ® (bosentan) as<br />
small as possible, TRACLEER ® may be prescribed only through the TRACLEER ® Access Program by calling 1 866 228<br />
3546. Adverse events can also be reported directly via this number.<br />
INDICATIONS AND USAGE: TRACLEER ® is indicated for the treatment of pulmonary arterial hypertension (WHO Group I) in patients<br />
with WHO Class III or IV symptoms, to improve exercise ability and decrease the rate of clinical worsening.<br />
CONTRAINDICATIONS: See BOX WARNING for CONTRAINDICATION to use in pregnancy.<br />
Pregnancy Category X. TRACLEER ® is expected to cause fetal harm if administered to pregnant women. Bosentan was teratogenic<br />
in rats given oral doses ≥ 60 mg/kg/day (twice the maximum recommended human oral dose of 125 mg, b.i.d., on a mg/m 2 basis). In<br />
an embryo-fetal toxicity study in rats, bosentan showed dose-dependent teratogenic effects, including malformations of the head,<br />
mouth, face and large blood vessels. Bosentan increased stillbirths and pup mortality at oral doses of 60 and 300 mg/kg/day (2 and 10<br />
times, respectively, the maximum recommended human dose on a mg/m 2 basis). Although birth defects were not observed in rabbits<br />
given oral doses of up to 1500 mg/kg/day, plasma concentrations of bosentan in rabbits were lower than those reached in the rat. The<br />
similarity of malformations induced by bosentan and those observed in endothelin-1 knockout mice and in animals treated with other<br />
endothelin receptor antagonists indicates that teratogenicity is a class effect of these drugs. There are no data on the use of TRACLEER ®<br />
in pregnant women. Pregnancy must be excluded before the start of treatment with TRACLEER ® and prevented thereafter by use of<br />
reliable contraception. It has been demonstrated that hormonal contraceptives, including oral, injectable, transdermal, and<br />
implantable contraceptives may not be reliable in the presence of TRACLEER ® and should not be used as the sole contraceptive<br />
method in patients receiving TRACLEER ® (see Drug Interactions: Hormonal Contraceptives, Including Oral, Injectable,<br />
Transdermal and Implantable Contraceptives). Input from a gynecologist or similar expert on adequate contraception should<br />
be sought as needed. TRACLEER ® should be started only in patients known not to be pregnant. For female patients of childbearing<br />
potential, a prescription for TRACLEER ® should not be issued by the prescriber unless the patient assures the prescriber that she is<br />
not sexually active or provides negative results from a urine or serum pregnancy test performed during the first 5 days of a normal<br />
menstrual period and at least 11 days after the last unprotected act of sexual intercourse. Follow-up urine or serum pregnancy tests<br />
should be obtained monthly in women of childbearing potential taking TRACLEER ® . The patient must be advised that if there is any<br />
delay in onset of menses or any other reason to suspect pregnancy, she must notify the physician immediately for pregnancy testing.<br />
If the pregnancy test is positive, the physician and patient must discuss the risk to the pregnancy and to the fetus.<br />
Cyclosporine A: Co-administration of cyclosporine A and bosentan resulted in markedly increased plasma concentrations of<br />
bosentan. Therefore, concomitant use of TRACLEER ® and cyclosporine A is contraindicated.<br />
Glyburide: An increased risk of liver enzyme elevations was observed in patients receiving glyburide concomitantly with bosentan.<br />
Therefore co-administration of glyburide and TRACLEER ® is contraindicated.<br />
Hypersensitivity: TRACLEER ® is also contraindicated in patients who are hypersensitive to bosentan or any component of<br />
the medication.<br />
WARNINGS: Potential Liver Injury (see BOX WARNING): Elevations in ALT or AST by more than 3 x ULN were observed in 11%<br />
of bosentan-treated patients (N = 658) compared to 2% of placebo-treated patients (N = 280). Three-fold increases were seen<br />
in 12% of 95 PAH patients on 125 mg b.i.d. and 14% of 70 PAH patients on 250 mg b.i.d. Eight-fold increases were seen in 2%<br />
of PAH patients on 125 mg b.i.d. and 7% of PAH patients on 250 mg b.i.d. Bilirubin increases to ≥3 x ULN were associated with<br />
aminotransferase increases in 2 of 658 (0.3%) of patients treated with bosentan. The combination of hepatocellular injury (increases<br />
in aminotransferases of > 3 x ULN) and increases in total bilirubin (≥ 3 x ULN) is a marker for potential serious liver injury. Elevations<br />
of AST and/or ALT associated with bosentan are dose-dependent, occur both early and late in treatment, usually progress slowly,<br />
are typically asymptomatic, and usually have been reversible after treatment interruption or cessation. Aminotransferase elevations<br />
also may reverse spontaneously while continuing treatment with TRACLEER ® . Liver aminotransferase levels must be measured prior<br />
to initiation of treatment and then monthly. If elevated aminotransferase levels are seen, changes in monitoring and treatment must<br />
be initiated. If liver aminotransferase elevations are accompanied by clinical symptoms of liver injury (such as nausea, vomiting,<br />
fever, abdominal pain, jaundice, or unusual lethargy or fatigue) or increases in bilirubin ≥ 2 x ULN, treatment should be stopped.<br />
There is no experience with the re-introduction of TRACLEER ® in these circumstances. Pre-existing Liver Impairment: Liver aminotransferase<br />
levels must be measured prior to initiation of treatment and then monthly. TRACLEER ® should generally be avoided in<br />
patients with moderate or severe liver impairment. In addition, TRACLEER ® should generally be avoided in patients with elevated<br />
aminotransferases (> 3 x ULN) because monitoring liver injury in these patients may be more difficult (see BOX WARNING).<br />
PRECAUTIONS: Hematologic Changes: Treatment with TRACLEER ® caused a dose-related decrease in hemoglobin and hematocrit.<br />
Hemoglobin levels should be monitored after 1 and 3 months of treatment and then every 3 months. The overall mean decrease<br />
in hemoglobin concentration for bosentan-treated patients was 0.9 g/dL (change to end of treatment). Most of this decrease of<br />
hemoglobin concentration was detected during the first few weeks of bosentan treatment and hemoglobin levels stabilized by<br />
4–12 weeks of bosentan treatment. In placebo-controlled studies of all uses of bosentan, marked decreases in hemoglobin (> 15%<br />
decrease from baseline resulting in values < 11 g/dL) were observed in 6% of bosentan-treated patients and 3% of placebotreated<br />
patients. In patients with pulmonary arterial hypertension treated with doses of 125 and 250 mg b.i.d., marked decreases<br />
in hemoglobin occurred in 3% compared to 1% in placebo-treated patients. A decrease in hemoglobin concentration by at least<br />
1 g/dL was observed in 57% of bosentan-treated patients as compared to 29% of placebo-treated patients. In 80% of those patients<br />
whose hemoglobin decreased by at least 1 g/dL, the decrease occurred during the first 6 weeks of bosentan treatment. During the<br />
course of treatment the hemoglobin concentration remained within normal limits in 68% of bosentan-treated patients compared<br />
to 76% of placebo patients. The explanation for the change in hemoglobin is not known, but it does not appear to be hemorrhage<br />
or hemolysis. It is recommended that hemoglobin concentrations be checked after 1 and 3 months, and every 3 months thereafter.<br />
If a marked decrease in hemoglobin concentration occurs, further evaluation should be undertaken to determine the cause and<br />
need for specific treatment. Fluid retention: In a placebo-controlled trial of patients with severe chronic heart failure, there was<br />
an increased incidence of hospitalization for CHF associated with weight gain and increased leg edema during the first 4-8 weeks<br />
of treatment with TRACLEER ® . In addition, there have been numerous post-marketing reports of fluid retention in patients with<br />
pulmonary hypertension, occurring within weeks after starting TRACLEER ® . Patients required intervention with a diuretic, fluid<br />
management, or hospitalization for decompensating heart failure. Pulmonary Veno-Occlusive Disease (PVOD): Should signs<br />
of pulmonary edema occur when TRACLEER ® is administered the possibility of associated PVOD should be considered and<br />
TRACLEER ® should be discontinued. Pulmonary Arterial Hypertension Associated with HIV Infection: There is limited clinical<br />
trial experience with the use of TRACLEER ® in patients with PAH associated with HIV infection who are treated concomitantly<br />
with antiretroviral medications. An interaction study between bosentan and lopinavir+ritonavir in healthy subjects showed<br />
increased plasma concentrations of bosentan and decreased concentrations of lopinavir+ritonavir (see PRECAUTIONS: Drug<br />
Interactions). Due to the potential for interactions related to the inducing effect of bosentan on CYP450, which could affect<br />
the efficacy of some antiretroviral therapies, patients should be monitored carefully regarding their HIV infection. Conversely,<br />
due to the inhibition of organic anion-transporting polypeptides (OATP) by ritonavir, there may be an increase in exposure to<br />
bosentan. The potential for an increased risk of hepatic toxicity and hematological adverse events cannot be excluded.<br />
Information for Patients: Patients are advised to consult the TRACLEER ® Medication Guide on the safe use of TRACLEER ® . The<br />
physician should discuss with the patient the importance of monthly monitoring of serum aminotransferases and urine or serum<br />
pregnancy testing and of avoidance of pregnancy. The physician should discuss options for effective contraception and measures<br />
to prevent pregnancy with their female patients. Input from a gynecologist or similar expert on adequate contraception should be<br />
sought as needed.<br />
Drug Interactions: Bosentan is metabolized by CYP2C9 and CYP3A4. Inhibition of these enzymes may increase the plasma concentration<br />
of bosentan (see ketoconazole). Concomitant administration of both a CYP2C9 inhibitor (such as fluconazole or amiodarone)<br />
and a CYP3A4 inhibitor (such as ketoconazole, itraconazole, or ritonavir) with bosentan will likely lead to large increases in plasma<br />
concentrations of bosentan. Co-administration of such combinations of a potent CYP2C9 inhibitor plus a CYP3A4 inhibitor with<br />
TRACLEER ® is not recommended. Bosentan is an inducer of CYP3A4 and CYP2C9. Consequently plasma concentrations of drugs<br />
metabolized by these two isozymes will be decreased when TRACLEER ® is co-administered. Bosentan had no relevant inhibitory<br />
effect on any CYP isozyme in vitro (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4). Consequently, TRACLEER ® is not expected to<br />
increase the plasma concentrations of drugs metabolized by these enzymes.<br />
Hormonal Contraceptives, Including Oral, Injectable, Transdermal, and Implantable Contraceptives: An interaction<br />
study demonstrated that co-administration of bosentan and the oral hormonal contraceptive Ortho-<strong>No</strong>vum ® produced<br />
average decreases of norethindrone and ethinyl estradiol levels of 14% and 31%, respectively. However, decreases in<br />
exposure were as much as 56% and 66%, respectively, in individual subjects. Therefore, hormonal contraceptives, including<br />
oral, injectable, transdermal, and implantable forms, may not be reliable when TRACLEER ® is co-administered. Women<br />
should practice additional methods of contraception and not rely on hormonal contraception alone when taking TRACLEER ® .<br />
Specific interaction studies have demonstrated the following:<br />
Cyclosporine A: During the first day of concomitant administration, trough concentrations of bosentan were increased by about<br />
30-fold. Steady-state bosentan plasma concentrations were 3- to 4-fold higher than in the absence of cyclosporine A. The<br />
concomitant administration of bosentan and cyclosporine A is contraindicated (see CONTRAINDICATIONS). Co-administration of<br />
bosentan decreased the plasma concentrations of cyclosporine A (a CYP3A4 substrate) by approximately 50%.<br />
Tacrolimus: Co-administration of tacrolimus and bosentan has not been studied in man. Co-administration of tacrolimus and<br />
bosentan resulted in markedly increased plasma concentrations of bosentan in animals. Caution should be exercised if tacrolimus<br />
and bosentan are used together.<br />
Glyburide: An increased risk of elevated liver aminotransferases was observed in patients receiving concomitant therapy with<br />
glyburide. Therefore, the concomitant administration of TRACLEER ® and glyburide is contraindicated, and alternative hypoglycemic<br />
agents should be considered (see CONTRAINDICATIONS). Co-administration of bosentan decreased the plasma concentrations<br />
of glyburide by approximately 40%. The plasma concentrations of bosentan were also decreased by approximately 30%. Bosentan<br />
is also expected to reduce plasma concentrations of other oral hypoglycemic agents that are predominantly metabolized by CYP2C9<br />
or CYP3A4. The possibility of worsened glucose control in patients using these agents should be considered.<br />
Ketoconazole: Co-administration of bosentan 125 mg b.i.d. and ketoconazole, a potent CYP3A4 inhibitor, increased the plasma<br />
concentrations of bosentan by approximately 2-fold. <strong>No</strong> dose adjustment of bosentan is necessary, but increased effects of bosentan<br />
should be considered.<br />
Simvastatin and Other Statins: Co-administration of bosentan decreased the plasma concentrations of simvastatin (a CYP3A4<br />
substrate), and its active -hydroxy acid metabolite, by approximately 50%. The plasma concentrations of bosentan were not affected.<br />
Bosentan is also expected to reduce plasma concentrations of other statins that have significant metabolism by CYP3A4, such<br />
as lovastatin and atorvastatin. The possibility of reduced statin efficacy should be considered. Patients using CYP3A4 metabolized<br />
statins should have cholesterol levels monitored after TRACLEER ® is initiated to see whether the statin dose needs adjustment.<br />
Warfarin: Co-administration of bosentan 500 mg b.i.d. for 6 days decreased the plasma concentrations of both S-warfarin (a<br />
CYP2C9 substrate) and R-warfarin (a CYP3A4 substrate) by 29 and 38%, respectively. Clinical experience with concomitant<br />
administration of bosentan and warfarin in patients with pulmonary arterial hypertension did not show clinically relevant<br />
changes in INR or warfarin dose (baseline vs. end of the clinical studies), and the need to change the warfarin dose during the<br />
trials due to changes in INR or due to adverse events was similar among bosentan- and placebo-treated patients.<br />
Digoxin, Nimodipine and Losartan: Bosentan has no significant pharmacokinetic interactions with digoxin and nimodipine, and<br />
losartan has no significant effect on plasma levels of bosentan.<br />
Sildenafil: In healthy subjects, co-administration of multiple doses of 125 mg b.i.d bosentan and 80 mg t.i.d. sildenafil<br />
resulted in a reduction of sildenafil plasma concentrations by 63% and increased bosentan plasma concentrations by 50%.<br />
A dose adjustment of neither drug is necessary. This recommendation holds true when sildenafil is used for the treatment of<br />
pulmonary arterial hypertension or erectile dysfunction.<br />
Iloprost: In a small, randomized, double-blind, placebo-controlled study (the STEP trial), 34 patients treated with bosentan 125<br />
mg bid for at least 16 weeks tolerated the addition of inhaled iloprost (up to 5 mcg 6 to 9 times per day during waking hours).<br />
The mean daily inhaled dose was 27 mcg and the mean number of inhalations per day was 5.6.<br />
Rifampicin: Coadministration of bosentan and rifampicin in normal volunteers resulted in a mean 6-fold increase in bosentan<br />
trough levels after the first concomitant dose, but about a 60% decrease in bosentan levels at steady-state. The effect of bosentan<br />
on rifampicin levels has not been assessed. When consideration of the potential benefits and known and unknown risks leads to<br />
concomitant use, measure LFTs weekly for the first 4 weeks before reverting to normal monitoring.<br />
Lopinavir and ritonavir: Co-administration of TRACLEER ® 125 mg twice daily and lopinavir+ritonavir 400 mg + 100 mg twice<br />
daily during 9.5 days in healthy subjects resulted in initial trough plasma concentrations of bosentan that were approximately<br />
48-fold higher than those measured after TRACLEER ® administered alone. At steady state, plasma concentrations of bosentan<br />
were approximately 5-fold higher than with TRACLEER ® administered alone. Inhibition by ritonavir of OATP-mediated<br />
uptake into hepatocytes, reducing the clearance of bosentan, most likely explains this interaction. After co-administration of<br />
TRACLEER ® , the plasma exposures to lopinavir and ritonavir at steady state decreased by approximately 14% and 17%,<br />
respectively. When TRACLEER ® is administered concomitantly with lopinavir+ritonavir or other ritonavir-boosted protease<br />
inhibitors, there should be appropriate monitoring of TRACLEER ® tolerability and ongoing HIV status (see PRECAUTIONS).<br />
Carcinogenesis, Mutagenesis, Impairment of Fertility: Two years of dietary administration of bosentan to mice produced<br />
an increased incidence of hepatocellular adenomas and carcinomas in males at doses as low as 450 mg/kg/day (about 8 times<br />
the maximum recommended human dose [MRHD] of 125 mg b.i.d., on a mg/m 2 basis). In the same study, doses greater than<br />
2000 mg/kg/day (about 32 times the MRHD) were associated with an increased incidence of colon adenomas in both males<br />
and females. In rats, dietary administration of bosentan for two years was associated with an increased incidence of brain<br />
astrocytomas in males at doses as low as 500 mg/kg/day (about 16 times the MRHD). In a comprehensive battery of in vitro<br />
tests (the microbial mutagenesis assay, the unscheduled DNA synthesis assay, the V-79 mammalian cell mutagenesis assay,<br />
and human lymphocyte assay) and an in vivo mouse micronucleus assay, there was no evidence for any mutagenic or clastogenic<br />
activity of bosentan. Impairment of Fertility/Testicular Function: Many endothelin receptor antagonists have profound<br />
effects on the histology and function of the testes in animals. These drugs have been shown to induce atrophy of the seminiferous<br />
tubules of the testes and to reduce sperm counts and male fertility in rats when administered for longer than 10 weeks.<br />
Where studied, testicular tubular atrophy and decreases in male fertility observed with endothelin receptor antagonists appear<br />
irreversible. In fertility studies in which male and female rats were treated with bosentan at oral doses of up to 1500<br />
mg/kg/day (50 times the MRHD on a mg/m 2 basis) or intravenous doses up to 40 mg/kg/day, no effects on sperm count, sperm<br />
motility, mating performance or fertility were observed. An increased incidence of testicular tubular atrophy was observed in<br />
rats given bosentan orally at doses as low as 125 mg/kg/ day (about 4 times the MRHD and the lowest doses tested) for two<br />
years but not at doses as high as 1500 mg/kg/day (about 50 times the MRHD) for 6 months. Effects on sperm count and motility<br />
were evaluated only in the much shorter duration fertility studies in which males had been exposed to the drug for 4-6 weeks.<br />
An increased incidence of tubular atrophy was not observed in mice treated for 2 years at doses up to 4500 mg/kg/day (about<br />
75 times the MRHD) or in dogs treated up to 12 months at doses up to 500 mg/kg/day (about 50 times the MRHD). There are<br />
no data on the effects of bosentan or other endothelin receptor antagonists on testicular function in man.<br />
Pregnancy, Teratogenic Effects: Category X (See CONTRAINDICATIONS).<br />
Special Populations: Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are<br />
excreted in human milk, breastfeeding while taking TRACLEER ® is not recommended. Pediatric Use: Safety and efficacy in pediatric<br />
patients have not been established. Use in Elderly Patients: Clinical experience with TRACLEER ® in subjects aged 65 or older has not<br />
included a sufficient number of such subjects to identify a difference in response between elderly and younger patients.<br />
ADVERSE REACTIONS: Adverse Events: See BOX WARNING for discussion of liver injury and PRECAUTIONS for discussion<br />
of hemoglobin and hematocrit abnormalities. Safety data on bosentan were obtained from 12 clinical studies (8 placebo-controlled<br />
and 4 open-label) in 777 patients with pulmonary arterial hypertension, and other diseases. Doses up to 8 times the currently<br />
recommended clinical dose (125 mg b.i.d.) were administered for a variety of durations. The exposure to bosentan in these trials<br />
ranged from 1 day to 4.1 years (N = 89 for 1 year; N = 61 for 1.5 years and N = 39 for more than 2 years). Exposure of<br />
pulmonary arterial hypertension patients (N = 235) to bosentan ranged from 1 day to 1.7 years (N = 126 more than 6 months and<br />
N = 28 more than 12 months). Treatment discontinuations due to adverse events other than those related to pulmonary hypertension<br />
during the clinical trials in patients with pulmonary arterial hypertension were more frequent on bosentan (5%; 8/165 patients) than<br />
on placebo (3%; 2/80 patients). In this database the only cause of discontinuations >1%, and occurring more often on bosentan was<br />
abnormal liver function. The adverse drug reactions that occurred in ≥ 3% of the bosentan-treated patients and were more common<br />
on bosentan in placebo-controlled trials in pulmonary arterial hypertension at doses of 125 or 250 mg b.i.d. are shown in Table 1:<br />
Table 1. Adverse events* occurring in ≥ 3% of patients treated with bosentan 125-250 mg b.i.d. and more common on bosentan in<br />
placebo-controlled studies in pulmonary arterial hypertension<br />
Adverse Event<br />
Bosentan (N = 165) Placebo (N = 80)<br />
<strong>No</strong>. % <strong>No</strong>. %<br />
Headache 36 22% 16 20%<br />
Nasopharyngitis 18 11% 6 8%<br />
Flushing 15 9% 4 5%<br />
Hepatic function abnormal 14 8% 2 3%<br />
Edema, lower limb 13 8% 4 5%<br />
Hypotension 11 7% 3 4%<br />
Palpitations 8 5% 1 1%<br />
Dyspepsia 7 4% 0 0%<br />
Edema 7 4% 2 3%<br />
Fatigue 6 4% 1 1%<br />
Pruritus 6 4% 0 0%<br />
*<strong>No</strong>te: only AEs with onset from start of treatment to 1 calendar day after end of treatment are included. All<br />
reported events (at least 3%) are included except those too general to be informative, and those not reasonably<br />
associated with the use of the drug because they were associated with the condition being treated or are very<br />
common in the treated population.<br />
In placebo-controlled studies of bosentan in pulmonary arterial hypertension and for other diseases (primarily chronic heart<br />
failure), a total of 677 patients were treated with bosentan at daily doses ranging from 100 mg to 2000 mg and 288 patients<br />
were treated with placebo. The duration of treatment ranged from 4 weeks to 6 months. For the adverse drug reactions that<br />
occurred in ≥ 3% of bosentan-treated patients, the only ones that occurred more frequently on bosentan than on placebo<br />
(≥ 2% difference) were headache (16% vs. 13%), flushing (7% vs. 2%), abnormal hepatic function (6% vs. 2%), leg edema<br />
(5% vs. 1%), and anemia (3% vs. 1%).<br />
Post-Marketing Experience: Hypersensitivity, Rash, Thrombocytopenia, Jaundice, Anemia requiring transfusion: There have<br />
been several post-marketing reports of angioneurotic edema associated with the use of bosentan. The onset of the reported cases<br />
occurred within a range of 8 hours to 21 days after starting therapy. Some patients were treated with an antihistamine and their<br />
signs of angioedema resolved without discontinuing TRACLEER ® . In the post-marketing period, in the setting of close monitoring,<br />
rare cases of unexplained hepatic cirrhosis were reported after prolonged (> 12 months) therapy with TRACLEER ® in patients with<br />
multiple co-morbidities and drug therapies. There have also been rare reports of liver failure. The contribution of TRACLEER ® in these<br />
cases could not be excluded (see BOX WARNING).<br />
Manufactured by:<br />
Marketed by:<br />
Patheon, Inc.<br />
Actelion Pharmaceuticals US, Inc.<br />
Mississauga, Ontario, L5N 7K9, CANADA<br />
South San Francisco, CA 94080, USA<br />
References for previous pages: 1. Data on file, Actelion Pharmaceuticals. 2. Rubin LJ, Badesch DB, Barst RJ, et al.<br />
Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896-903. 3. Channick RN, Simonneau G,<br />
Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension:<br />
a randomised placebo-controlled study. Lancet. 2001;358:1119-1123.<br />
© 2009 Actelion Pharmaceuticals US, Inc. All rights reserved. 07 354 01 03 0209<br />
TRACLEER<br />
se of<br />
U ® s<br />
equire<br />
r s<br />
seriou<br />
for<br />
potential<br />
1)<br />
concerns:<br />
tention to two significant<br />
tt<br />
a l<br />
potentia<br />
and 2)<br />
injury<br />
liver<br />
bosentan decreased the plasma<br />
acrolimus:<br />
Ta<br />
n<br />
administratio<br />
o<br />
C<br />
by approxima<br />
cyclosporine A (a CYP3A4 substrate)<br />
concentrations of<br />
adm<br />
Co<br />
man<br />
in<br />
studied<br />
been<br />
not<br />
has<br />
bosentan<br />
and<br />
tacrolimus<br />
f<br />
o<br />
ately 50%.<br />
and<br />
tacrolimus<br />
of<br />
inistration<br />
m<br />
TRACLEER<br />
se of<br />
U<br />
s<br />
require<br />
damage to a fetus.<br />
inj<br />
liver<br />
Potential<br />
ARNING:<br />
WA<br />
RACLEER<br />
T ® 3<br />
least<br />
at<br />
causes<br />
accom<br />
patients,<br />
11% of<br />
about<br />
serious<br />
potential<br />
for<br />
marker<br />
and then monthly (see<br />
ment<br />
case<br />
close monitoring, rare<br />
ith TRACLEER<br />
w ® w<br />
in patients<br />
TR<br />
The contribution of<br />
failure.<br />
ini<br />
the<br />
case<br />
one<br />
least<br />
at<br />
In<br />
aminotransferases and biliru<br />
TRAC<br />
discontinuation of<br />
ter<br />
aft<br />
t<br />
the duration of<br />
schedule for<br />
aminotransferases accompa<br />
Elevations in aminotransferases<br />
elevated aminotransferases<br />
elevations<br />
aminotransferase<br />
fever, abdominal pain, jaundice,<br />
be stopped. There is no experience with the re-introduction of TRACLEER<br />
:<br />
ION:<br />
ATI<br />
DICA<br />
AIND<br />
RA<br />
ONTR<br />
C<br />
a<br />
Pregn<br />
ha<br />
as this effect<br />
women,<br />
nant<br />
b<br />
must<br />
pregnancy<br />
herefore<br />
T<br />
serious<br />
for<br />
potential<br />
1)<br />
concerns:<br />
tention to two significant<br />
att<br />
jury<br />
aminotran<br />
liver<br />
elevation of<br />
ULN)<br />
normal;<br />
of<br />
limit<br />
3-fold (upper<br />
Bec<br />
cases.<br />
of<br />
number<br />
elevated bilirubin in a small<br />
mpanied by<br />
measured<br />
be<br />
must<br />
levels<br />
serum aminotransferase<br />
injury,<br />
liver<br />
(see WARNINGS: Potential Liver Injury). In the post-marketing<br />
prolon<br />
ter<br />
aft<br />
reported<br />
were<br />
cirrhosis<br />
hepatic<br />
unexplained<br />
of<br />
s<br />
There have als<br />
with multiple co-morbidities and drug therapies.<br />
ACLEER<br />
R ® .<br />
be excluded<br />
in these cases could not<br />
included<br />
treatment)<br />
of<br />
months<br />
20<br />
><br />
ter<br />
(aft<br />
presentation<br />
itial<br />
whic<br />
of<br />
all<br />
bin levels accompanied by non-specific symptoms,<br />
LEER<br />
C ® c<br />
adheren<br />
strict<br />
This case reinforces the importance of<br />
.<br />
which includes stoppin<br />
algorithm,<br />
and the treatment<br />
treatment<br />
dysfunction.<br />
liver<br />
symptoms of<br />
nied by signs or<br />
require close attention. TRACLEER<br />
aminotransferases ® be<br />
should generally<br />
aminotransferases (> 3 x ULN) at baseline because monitoring liver injury may<br />
(such<br />
injury<br />
liver<br />
of<br />
symptoms<br />
clinical<br />
by<br />
accompanied<br />
are<br />
elevations<br />
or unusual lethargy or fatigue) or increases in bilirubin<br />
jaundice,<br />
≥<br />
be stopped. There is no experience with the re-introduction of TRACLEER ® in these circumstances.<br />
TRACLEER<br />
ncy.<br />
a ® i<br />
b<br />
is very likely to produce major<br />
(bosentan)<br />
is administered to animals (s<br />
as been seen consistently when it<br />
TRACLEER<br />
with<br />
treatment<br />
of<br />
start<br />
the<br />
before<br />
excluded<br />
e<br />
b ® n<br />
a<br />
potential<br />
and 2)<br />
,<br />
injury,<br />
liver<br />
in<br />
T and AST)<br />
(ALT<br />
nsferases<br />
are a<br />
cause these changes<br />
treat<br />
of<br />
initiation<br />
to<br />
prior<br />
d -<br />
post-marketing period, in the setting of<br />
therapy<br />
months)<br />
(>12<br />
nged<br />
liver<br />
so been rare reports of<br />
in<br />
elevations<br />
pronounced<br />
time<br />
h resolved slowly over<br />
e to the monthly monitoring<br />
TRACLEER<br />
g ® f<br />
with a rise o<br />
be avoided in patients with<br />
may be more difficult. If liver<br />
vomiting,<br />
nausea,<br />
as<br />
(such<br />
≥<br />
should<br />
2 x ULN, treatment<br />
in these circumstances.<br />
used by preg<br />
th defects if<br />
r -<br />
TIONS).<br />
see CONTRAINDICAT<br />
by<br />
thereafter<br />
prevented<br />
nd<br />
acrolimus:<br />
Ta<br />
n<br />
Co-administratio<br />
i<br />
bosentan resulted in markedly<br />
and bosentan are used together<br />
lyburide:<br />
G<br />
o<br />
risk<br />
increased<br />
An<br />
the concom<br />
Therefore,<br />
glyburide.<br />
considered (se<br />
should be<br />
agents<br />
glyburide by approximately 40<br />
of<br />
is also expected to reduce plasm<br />
wo<br />
The possibility of<br />
CYP3A4.<br />
or<br />
etoconazole:<br />
K<br />
t<br />
Co-administra<br />
bosentan by a<br />
concentrations of<br />
should be considered.<br />
Statin<br />
Simvastatin and Other<br />
and its active<br />
ubstrate),<br />
s<br />
o<br />
-hydr<br />
also expected t<br />
Bosentan is<br />
ed.<br />
T<br />
lovastatin and atorvastatin.<br />
as<br />
statins should have cholesterol<br />
arfarin:<br />
W<br />
of<br />
Co-administration<br />
CYP2C9 substrate) and R-warfarin<br />
administration of bosentan and<br />
changes in INR or warfarin dose<br />
trials due to changes in INR or due to adverse events was similar among bosentan- and placebo-treated patients.<br />
Nimodipine and Los<br />
igoxin<br />
D<br />
Co-adm<br />
man.<br />
in<br />
studied<br />
been<br />
not<br />
has<br />
bosentan<br />
and<br />
tacrolimus<br />
of<br />
Caution sho<br />
bosentan in animals.<br />
of<br />
ncreased plasma concentrations<br />
r.<br />
receiv<br />
patients<br />
in<br />
observed<br />
was<br />
aminotransferases<br />
liver<br />
elevated<br />
of<br />
TRACLEER<br />
administration of<br />
itant<br />
m ® d<br />
and glyburide is contraindicate<br />
e<br />
e<br />
S<br />
ION<br />
TI<br />
ONTRAINDICAT<br />
C<br />
s<br />
bosentan decrea<br />
Co-administration of<br />
).<br />
bosentan were also decreased by<br />
The plasma concentrations of<br />
0%.<br />
are predomi<br />
hypoglycemic agents that<br />
oral<br />
other<br />
ma concentrations of<br />
in patients using these agents should be con<br />
orsened glucose control<br />
i<br />
CYP3A4<br />
potent<br />
a<br />
ketoconazole,<br />
and<br />
b.i.d.<br />
mg<br />
125<br />
bosentan<br />
of<br />
ion<br />
but<br />
bosentan is necessary,<br />
of<br />
<strong>No</strong> dose adjustment<br />
pproximately 2-fold.<br />
s:<br />
n<br />
t<br />
concentra<br />
plasma<br />
the<br />
decreased<br />
bosentan<br />
of<br />
Co-administration<br />
The plasma concentration<br />
by approximately 50%.<br />
oxy acid metabolite,<br />
have significant<br />
that<br />
statins<br />
other<br />
of<br />
o reduce plasma concentrations<br />
Patien<br />
should be considered.<br />
reduced statin efficacy<br />
of<br />
The possibility<br />
TRACLEER<br />
evels monitored after<br />
l ® a<br />
the st<br />
is initiated to see whether<br />
of bosentan 500 mg b.i.d. for 6 days decreased the plasma concentrations<br />
R-warfarin (a CYP3A4 substrate) by 29 and 38%, respectively. Clinical<br />
and warfarin in patients with pulmonary arterial hypertension did<br />
dose (baseline vs. end of the clinical studies), and the need to change<br />
trials due to changes in INR or due to adverse events was similar among bosentan- and placebo-treated patients.<br />
artan:<br />
s<br />
t<br />
pharmacokinetic interactions wi<br />
osentan has no significant<br />
B<br />
and<br />
tacrolimus<br />
of<br />
ministration<br />
tacrolimus<br />
ould be exercised if<br />
with<br />
therapy<br />
concomitant<br />
ving<br />
and alternative hypoglycemic<br />
,<br />
concentrations<br />
sed the plasma<br />
Bosentan<br />
approximately 30%.<br />
nantly metabolized by CYP2C9<br />
sidered.<br />
plasma<br />
the<br />
increased<br />
nhibitor,<br />
bosentan<br />
increased effects of<br />
t<br />
CYP3A4<br />
(a<br />
simvastatin<br />
of<br />
ions<br />
affect<br />
bosentan were not<br />
s of<br />
n -<br />
such<br />
CYP3A4,<br />
metabolism by<br />
t<br />
using CYP3A4 metabolized<br />
nts<br />
tin dose needs adjustment.<br />
concentrations of both S-warfarin (a<br />
experience with concomitant<br />
not show clinically relevant<br />
change the warfarin dose during the<br />
trials due to changes in INR or due to adverse events was similar among bosentan- and placebo-treated patients.<br />
and<br />
h digoxin and nimodipine<br />
t<br />
b<br />
must<br />
pregnancy<br />
Therefore,<br />
method<br />
reliable<br />
a<br />
of<br />
use<br />
the<br />
implantable contraceptives s<br />
TRACLE<br />
receiving<br />
patients<br />
in<br />
contracep<br />
forms of<br />
additional<br />
inj<br />
liver<br />
potential<br />
of<br />
Because<br />
TRACLEER<br />
possible,<br />
as<br />
small<br />
Adverse events can als<br />
3546.<br />
IONS AND USAGE:<br />
TI<br />
NDICAT<br />
I<br />
T<br />
IV sympto<br />
or<br />
with WHO Class III<br />
TIONS:<br />
RAINDICAT<br />
ONTR<br />
C<br />
e<br />
e<br />
S<br />
B<br />
y X.<br />
regnancy Category<br />
P<br />
L<br />
TRAC<br />
doses ≥ 60 mg/<br />
in rats given oral<br />
in<br />
study<br />
toxicity<br />
an embryo-fetal<br />
face and large blood vess<br />
mouth,<br />
the maximum<br />
respectively,<br />
times,<br />
doses of up to 1500 mg<br />
given oral<br />
malformations induc<br />
similarity of<br />
endothelinreceptorantagonistsi<br />
Pregnancy m<br />
women.<br />
in pregnant<br />
reliable contraception. It has been<br />
may<br />
implantable contraceptives<br />
TR<br />
i<br />
i<br />
i<br />
i<br />
d<br />
h<br />
TRACLEER<br />
with<br />
treatment<br />
of<br />
start<br />
the<br />
before<br />
excluded<br />
e<br />
b ® n<br />
a<br />
in<br />
oral,<br />
including<br />
contraceptives,<br />
Hormonal<br />
contraception.<br />
of<br />
contraception because<br />
be used as the sole means of<br />
hould not<br />
ER<br />
E ® c<br />
fe<br />
eff<br />
Therefore,<br />
Interactions).<br />
Drug<br />
PRECAUTIONS:<br />
(see<br />
Monthly pregnancy tests should be ob<br />
be practiced.<br />
ption must<br />
to<br />
exposure<br />
fetal<br />
of<br />
chance<br />
the<br />
make<br />
to<br />
fort<br />
eff<br />
an<br />
in<br />
and<br />
jury<br />
R ®<br />
R<br />
through the TRACLEE<br />
be prescribed only<br />
ay<br />
m ® r<br />
P<br />
Access<br />
so be reported directly via this number.<br />
ACLEER<br />
R ® n<br />
hyperte<br />
is indicated for the treatment of pulmonary arterial<br />
wors<br />
clinical<br />
to improve exercise ability and decrease the rate of<br />
ms,<br />
ARNING<br />
OX WA<br />
B<br />
r<br />
o<br />
f<br />
N<br />
IO<br />
TI<br />
RAINDICAT<br />
ONTR<br />
C .<br />
to use in pregnancy<br />
EER<br />
L ® m<br />
wo<br />
administered to pregnant<br />
harm if<br />
is expected to cause fetal<br />
125 m<br />
dose of<br />
/kg/day (twice the maximum recommended human oral<br />
includin<br />
effects,<br />
teratogenic<br />
bosentan showed dose-dependent<br />
n rats,<br />
doses of 6<br />
Bosentan increased stillbirths and pup mortality at oral<br />
sels.<br />
recommended human dose on a mg/m<br />
m 2 t<br />
Although birth defec<br />
basis).<br />
plasma concentrations of bosentan in rabbits were lower tha<br />
g/kg/day,<br />
mice an<br />
ced by bosentan and those observed in endothelin-1 knockout<br />
ndicatesthatteratogenicityisaclasseffectofthesedrugs.Thereareno<br />
with TRACLEER<br />
treatment<br />
of<br />
be excluded before the start<br />
ust<br />
m ® d<br />
an<br />
been demonstrated that hormonal contraceptives, including oral,<br />
TRACLEER<br />
of<br />
presence<br />
the<br />
in<br />
reliable<br />
be<br />
not<br />
y ® u<br />
be<br />
not<br />
should<br />
and<br />
ACLEER<br />
R ® ( i<br />
C<br />
l<br />
H<br />
i<br />
D I<br />
by<br />
thereafter<br />
prevented<br />
nd<br />
and<br />
transdermal,<br />
njectable,<br />
be effective<br />
e these may not<br />
through<br />
contraception<br />
ctive<br />
tained.<br />
RACLEER<br />
T ® s<br />
a<br />
(bosentan)<br />
228<br />
866<br />
calling 1<br />
rogram by<br />
in patients<br />
nsion (WHO Group I)<br />
ening.<br />
Bosentan was teratogenic<br />
men.<br />
on a mg/m<br />
b.i.d.,<br />
g,<br />
m 2 n<br />
I<br />
basis).<br />
the head,<br />
of<br />
ng malformations<br />
0 and 300 mg/kg/day (2 and 10<br />
observed in rabbits<br />
ts were not<br />
The<br />
an those reached in the rat.<br />
d in animals treated with other<br />
dataontheuseofTRACLEER<br />
o ®<br />
by use of<br />
prevented thereafter<br />
injectable, transdermal, and<br />
contraceptive<br />
sole<br />
the<br />
as<br />
used<br />
bl<br />
j<br />
I<br />
l<br />
O<br />
di<br />
l<br />
I<br />
Nimodipine and Los<br />
Digoxin,<br />
effec<br />
losartan has no significant<br />
Sildenafil:<br />
subjects,<br />
healthy<br />
In<br />
resulted in a reduction of sildenafil<br />
A dose adjustment of neither drug<br />
pulmonary arterial hypertension or erectile dysfunction.<br />
Iloprost:<br />
randomized,<br />
In a small,<br />
mg bid for at least 16 weeks tolerated<br />
The mean daily inhaled dose was 27 mcg and the mean number of inhalations per day was 5.6.<br />
ifampicin:<br />
R<br />
n<br />
Coadministratio<br />
conc<br />
the first<br />
trough levels after<br />
bee<br />
not<br />
has<br />
levels<br />
rifampicin<br />
on<br />
s<br />
measure LFTs<br />
use,<br />
concomitant<br />
and ritonavir:<br />
Lopinavir<br />
Co-administration<br />
daily during 9.5 days in healthy<br />
48-fold higher than those measured<br />
tan were approximately 5-fold<br />
uptake into hepatocytes, reducing<br />
TRACLEER ®<br />
exposures<br />
plasma<br />
the<br />
,<br />
TRACLEER<br />
When<br />
respectively.<br />
inhibitors, there should be appropriate monitoring of TRACLEER<br />
Carcinogenesis, Mutagenesis,<br />
an increased incidence of hepatocellular<br />
artan:<br />
s<br />
t<br />
pharmacokinetic interactions wi<br />
Bosentan has no significant<br />
bosentan.<br />
on plasma levels of<br />
ct<br />
bosentan<br />
b.i.d<br />
mg<br />
125<br />
of<br />
doses<br />
multiple<br />
of<br />
co-administration<br />
subjects,<br />
sildenafil plasma concentrations by 63% and increased bosentan plasma<br />
drug is necessary. This recommendation holds true when sildenafil<br />
pulmonary arterial hypertension or erectile dysfunction.<br />
randomized, double-blind, placebo-controlled study (the STEP trial), 34 patients<br />
tolerated the addition of inhaled iloprost (up to 5 mcg 6 to 9 times per<br />
The mean daily inhaled dose was 27 mcg and the mean number of inhalations per day was 5.6.<br />
mea<br />
a<br />
in<br />
resulted<br />
volunteers<br />
normal<br />
in<br />
rifampicin<br />
and<br />
bosentan<br />
of<br />
steady<br />
a 60% decrease in bosentan levels at<br />
about<br />
but<br />
dose,<br />
comitant<br />
know<br />
and<br />
benefits<br />
potential<br />
the<br />
of<br />
consideration<br />
When<br />
assessed.<br />
en<br />
monitoring.<br />
4 weeks before reverting to normal<br />
the first<br />
weekly for<br />
of TRACLEER<br />
Co-administration ® lopinavir+ritonavir<br />
125 mg twice daily and<br />
healthy subjects resulted in initial trough plasma concentrations of bosentan<br />
after TRACLEER<br />
measured ® plasma<br />
administered alone. At steady state,<br />
higher than with TRACLEER<br />
5-fold ® by<br />
administered alone. Inhibition<br />
reducing the clearance of bosentan, most likely explains this interaction.<br />
approximately<br />
by<br />
decreased<br />
state<br />
steady<br />
at<br />
ritonavir<br />
and<br />
lopinavir<br />
to<br />
exposures<br />
TRACLEER ®<br />
other<br />
or<br />
lopinavir+ritonavir<br />
with<br />
concomitantly<br />
administered<br />
is<br />
inhibitors, there should be appropriate monitoring of TRACLEER ® tolerability and ongoing HIV status (see<br />
Impairment of Fertility:<br />
Mutagenesis,<br />
administration<br />
wo years of dietary<br />
Two<br />
hepatocellular adenomas and carcinomas in males at doses as low as 450<br />
and<br />
th digoxin and nimodipine,<br />
sildenafil<br />
t.i.d.<br />
mg<br />
80<br />
and<br />
bosentan<br />
plasma concentrations by 50%.<br />
sildenafil is used for the treatment of<br />
patients treated with bosentan 125<br />
per day during waking hours).<br />
bosentan<br />
in<br />
increase<br />
6-fold<br />
n<br />
bosentan<br />
of<br />
The effect<br />
y-state.<br />
to<br />
leads<br />
risks<br />
unknown<br />
and<br />
wn<br />
lopinavir+ritonavir 400 mg + 100 mg twice<br />
bosentan that were approximately<br />
concentrations of bosen<br />
plasma -<br />
by ritonavir of OATP-mediated<br />
interaction. After co-administration of<br />
17%,<br />
and<br />
14%<br />
approximately<br />
protease<br />
ritonavir-boosted<br />
other<br />
tolerability and ongoing HIV status (see PRECAUTIONS).<br />
of bosentan to mice produced<br />
450 mg/kg/day (about 8 times<br />
TR<br />
receiving<br />
patients<br />
in<br />
method<br />
and Implantable<br />
ransdermal<br />
Tr<br />
TRACLEER<br />
needed.<br />
as<br />
be sought<br />
TRA<br />
a prescription for<br />
potential,<br />
n<br />
provides<br />
active or<br />
sexually<br />
not<br />
11<br />
least<br />
period and at<br />
menstrual<br />
should be obtained monthly in w<br />
any o<br />
menses or<br />
of<br />
delay in onset<br />
is positive,<br />
the pregnancy test<br />
If<br />
A<br />
yclosporine<br />
C<br />
r<br />
Co-administ<br />
:<br />
concomitan<br />
Therefore,<br />
bosentan.<br />
lyburide<br />
G<br />
f<br />
An increased risk o<br />
:<br />
g<br />
Therefore co-administration of<br />
Hypersensitivity TRACLEER<br />
: ®<br />
the medication.<br />
ARNINGS:<br />
WA<br />
j<br />
Inj<br />
er<br />
Live<br />
al<br />
Potentia<br />
(N<br />
patients<br />
bosentan-treated<br />
of<br />
1<br />
on<br />
PAH patients<br />
95<br />
12% of<br />
in<br />
b.i.d<br />
mg<br />
125<br />
on<br />
AH patients<br />
PA<br />
of<br />
aminotransferase increases in 2<br />
> 3 x UL<br />
in aminotransferases of<br />
T associated w<br />
ALT<br />
AST and/or<br />
of<br />
and<br />
re typically asymptomatic<br />
a<br />
ACLEER<br />
R ® e<br />
se<br />
( ,<br />
Contraceptives<br />
Hormonal<br />
Interactions:<br />
Drug<br />
Contraceptives<br />
e<br />
a<br />
on<br />
expert<br />
similar<br />
or<br />
gynecologist<br />
from a<br />
Input<br />
).<br />
R ®<br />
e<br />
f<br />
For<br />
to be pregnant.<br />
known not<br />
in patients<br />
should be started only<br />
LEER<br />
C ® s<br />
as<br />
unless the patient<br />
be issued by the prescriber<br />
should not<br />
performed duri<br />
test<br />
serum pregnancy<br />
from a urine or<br />
negative results<br />
Follow-up u<br />
intercourse.<br />
sexual<br />
of<br />
unprotected act<br />
the last<br />
days after<br />
taking TRACLEER<br />
childbearing potential<br />
omen of<br />
w ® t<br />
mus<br />
The patient<br />
.<br />
notify the physician imm<br />
she must<br />
pregnancy,<br />
reason to suspect<br />
other<br />
discuss the risk to the pregnancy and<br />
must<br />
the physician and patient<br />
increas<br />
markedly<br />
in<br />
resulted<br />
bosentan<br />
and<br />
A<br />
cyclosporine<br />
of<br />
ation<br />
TRACLEER<br />
use of<br />
t<br />
n ® .<br />
and cyclosporine A is contraindicated<br />
enzyme elevations was observed in patients receiving glyburide<br />
liver<br />
lyburide and TRACLEER<br />
g ® .<br />
is contraindicated<br />
® bosentan<br />
is also contraindicated in patients who are hypersensitive to<br />
y<br />
ury<br />
j<br />
e<br />
se<br />
( G<br />
ARNIN<br />
OX WA<br />
B 3<br />
more than<br />
AST by<br />
T or<br />
in ALT<br />
Elevations<br />
):<br />
Th<br />
280).<br />
(N =<br />
patients<br />
placebo-treated<br />
2% of<br />
to<br />
compared<br />
658)<br />
N =<br />
Eight-fo<br />
b.i.d.<br />
mg<br />
250<br />
on<br />
PAH patients<br />
70<br />
14% of<br />
and<br />
b.i.d.<br />
mg<br />
25<br />
≥3<br />
to<br />
increases<br />
Bilirubin<br />
b.i.d.<br />
mg<br />
250<br />
on<br />
AH patients<br />
PA<br />
7% of<br />
and<br />
d.<br />
h<br />
The combination of<br />
patients treated with bosentan.<br />
of<br />
658 (0.3%)<br />
of<br />
potential<br />
for<br />
is a marker<br />
bilirubin (≥ 3 x ULN)<br />
and increases in total<br />
N)<br />
and late in treatm<br />
both early<br />
occur<br />
with bosentan are dose-dependent,<br />
cessation<br />
interruption or<br />
treatment<br />
sually have been reversible after<br />
u<br />
Injectable,<br />
Oral,<br />
Including<br />
should<br />
contraception<br />
dequate<br />
childbearing<br />
of<br />
emale patients<br />
she is<br />
that<br />
sures the prescriber<br />
a normal<br />
of<br />
5 days<br />
ng the first<br />
serum pregnancy tests<br />
urine or<br />
there is any<br />
if<br />
be advised that<br />
pregnancy testing.<br />
ediately for<br />
to the fetus.<br />
of<br />
concentrations<br />
plasma<br />
sed<br />
concomitantly with bosentan.<br />
bosentan or any component of<br />
ULN were observed in 11%<br />
x<br />
seen<br />
were<br />
increases<br />
ree-fold<br />
2%<br />
in<br />
seen<br />
were<br />
increases<br />
ld<br />
with<br />
associated<br />
ULN were<br />
x<br />
3<br />
injury (increases<br />
hepatocellular<br />
injury.<br />
erious liver<br />
s<br />
s<br />
Elevation<br />
slowly,<br />
progress<br />
usually<br />
ment,<br />
Aminotransferase elevations<br />
n<br />
an increased incidence of hepatocellular<br />
the maximum recommended human<br />
2000 mg/kg/day (about 32 times<br />
and females. In rats, dietary administration<br />
astrocytomas in males at doses<br />
tests (the microbial mutagenesis<br />
and human lymphocyte assay)<br />
activity of bosentan.<br />
genic<br />
Impairment<br />
effects on the histology and function<br />
erous tubules of the testes and<br />
Where studied, testicular tubular<br />
pear irreversible. In fertility studies<br />
mg/kg/day (50 times the MRHD<br />
motility, mating performance or<br />
rats given bosentan orally at doses<br />
years but not at doses as high as<br />
were evaluated only in the much<br />
An increased incidence of tubular<br />
75 times the MRHD) or in dogs<br />
no data on the effects of bosentan or other endothelin receptor antagonists on testicular function in man.<br />
eratogenic Effe<br />
Te<br />
,<br />
Pregnancy,<br />
Populations:<br />
pecial<br />
S<br />
g<br />
n<br />
sin<br />
Nurs<br />
breastfe<br />
excreted in human milk,<br />
been establish<br />
atients have not<br />
p<br />
hepatocellular adenomas and carcinomas in males at doses as low as 450<br />
dose [MRHD] of 125 mg b.i.d., on a mg/m<br />
human 2 same<br />
basis). In the<br />
times the MRHD) were associated with an increased incidence of colon<br />
administration of bosentan for two years was associated with an<br />
doses as low as 500 mg/kg/day (about 16 times the MRHD). In a comprehensive<br />
mutagenesis assay, the unscheduled DNA synthesis assay, the V-79 mammalian<br />
and an in vivo mouse micronucleus assay, there was no evidence<br />
Fertility/Testicular Function:<br />
of Fertility/Testicular<br />
Impairment<br />
receptor<br />
Many endothelin<br />
function of the testes in animals. These drugs have been shown to induce<br />
and to reduce sperm counts and male fertility in rats when administered<br />
tubular atrophy and decreases in male fertility observed with endothelin<br />
studies in which male and female rats were treated with bosentan<br />
on a mg/m<br />
MRHD 2 effects<br />
basis) or intravenous doses up to 40 mg/kg/day, no<br />
or fertility were observed. An increased incidence of testicular tubular<br />
doses as low as 125 mg/kg/ day (about 4 times the MRHD and the<br />
as 1500 mg/kg/day (about 50 times the MRHD) for 6 months. Effects<br />
much shorter duration fertility studies in which males had been exposed<br />
tubular atrophy was not observed in mice treated for 2 years at doses<br />
dogs treated up to 12 months at doses up to 500 mg/kg/day (about 50<br />
no data on the effects of bosentan or other endothelin receptor antagonists on testicular function in man.<br />
Category X (<br />
ts:<br />
c<br />
e<br />
e<br />
S .<br />
TIONS)<br />
RAINDICAT<br />
CONTR<br />
s:<br />
hers<br />
Moth<br />
g<br />
m<br />
human<br />
in<br />
excreted<br />
is<br />
drug<br />
this<br />
whether<br />
known<br />
not<br />
is<br />
It<br />
eding while taking TRACLEER<br />
e ® .<br />
recommended<br />
s not<br />
i :<br />
c Use<br />
ric<br />
atr<br />
ia<br />
edi<br />
P<br />
S<br />
ed<br />
h :<br />
ents<br />
atie<br />
y Pa<br />
erly<br />
de<br />
n Eld<br />
se in<br />
Us<br />
R<br />
experience with TRACLEE<br />
linical<br />
C ® u<br />
n s<br />
i<br />
450 mg/kg/day (about 8 times<br />
same study, doses greater than<br />
colon adenomas in both males<br />
increased incidence of brain<br />
comprehensive battery of in vitro<br />
mammalian cell mutagenesis assay,<br />
for any mutagenic or clasto<br />
evidence -<br />
receptor antagonists have profound<br />
atrophy of the seminif<br />
induce -<br />
administered for longer than 10 weeks.<br />
receptor antagonists ap<br />
endothelin -<br />
bosentan at oral doses of up to 1500<br />
effects on sperm count, sperm<br />
tubular atrophy was observed in<br />
lowest doses tested) for two<br />
Effects on sperm count and motility<br />
exposed to the drug for 4-6 weeks.<br />
up to 4500 mg/kg/day (about<br />
50 times the MRHD). There are<br />
no data on the effects of bosentan or other endothelin receptor antagonists on testicular function in man.<br />
are<br />
drugs<br />
many<br />
Because<br />
milk.<br />
Safety and efficacy in pediatric<br />
has not<br />
older<br />
jects aged 65 or<br />
bj<br />
and<br />
are typically asymptomatic,<br />
also may reverse spontaneously<br />
and the<br />
treatment<br />
to initiation of<br />
aminotransf<br />
liver<br />
If<br />
initiated.<br />
be<br />
jaundice,<br />
pain,<br />
abdominal<br />
fever,<br />
no experience with the<br />
There is<br />
be meas<br />
must<br />
transferase levels<br />
sever<br />
moderate or<br />
with<br />
patients<br />
b<br />
aminotransferases (> 3 x ULN)<br />
RECAUTIONS:<br />
P<br />
C<br />
c<br />
ogic<br />
ematolo<br />
He<br />
Hemoglobin levels should be<br />
crit.<br />
for<br />
concentration<br />
hemoglobin<br />
in<br />
was<br />
concentration<br />
hemoglobin<br />
bosentan treatm<br />
4–12 weeks of<br />
resultin<br />
from baseline<br />
decrease<br />
wit<br />
patients<br />
In<br />
patients.<br />
treated<br />
3% c<br />
in<br />
occurred<br />
hemoglobin<br />
in<br />
b<br />
1 g/dL was observed in 57% of<br />
whose hemoglobin decreased by<br />
hemogl<br />
the<br />
treatment<br />
of<br />
course<br />
The<br />
placebo patients.<br />
to 76% of<br />
recommended<br />
is<br />
It<br />
hemolysis.<br />
or<br />
hemog<br />
in<br />
decrease<br />
marked<br />
a<br />
If<br />
treatment<br />
specific<br />
for<br />
eed<br />
n<br />
u<br />
lu<br />
Fl<br />
cessation<br />
interruption or<br />
treatment<br />
usually have been reversible after<br />
with TRACLEER<br />
hile continuing treatment<br />
w ® e<br />
aminotransferas<br />
Liver<br />
.<br />
changes in m<br />
elevated aminotransferase levels are seen,<br />
If<br />
en monthly.<br />
inju<br />
liver<br />
of<br />
symptoms<br />
clinical<br />
by<br />
accompanied<br />
are<br />
elevations<br />
erase<br />
ULN,<br />
x<br />
2<br />
≥<br />
bilirubin<br />
in<br />
increases<br />
or<br />
fatigue)<br />
or<br />
lethargy<br />
unusual<br />
or<br />
,<br />
TRACLEER<br />
e-introduction of<br />
r ® .<br />
n these circumstances<br />
i<br />
L<br />
ng<br />
stin<br />
xis<br />
e-ex<br />
re<br />
Pr<br />
TRACLEER<br />
and then monthly.<br />
treatment<br />
to initiation of<br />
ured prior<br />
s<br />
® s<br />
TRACLEER<br />
In addition,<br />
impairment.<br />
e liver<br />
r ® o<br />
be av<br />
should generally<br />
injury in these patients may be more difficult<br />
ecause monitoring liver<br />
es:<br />
hange<br />
Ch<br />
R<br />
TRACLEE<br />
with<br />
reatment<br />
T ® s<br />
decrea<br />
dose-related<br />
a<br />
caused<br />
and then every 3 mont<br />
treatment<br />
1 and 3 months of<br />
e monitored after<br />
treatme<br />
of<br />
end<br />
to<br />
(change<br />
g/dL<br />
0.9<br />
was<br />
patients<br />
bosentan-treated<br />
r<br />
he<br />
and<br />
treatment<br />
bosentan<br />
of<br />
few weeks<br />
first<br />
the<br />
during<br />
detected<br />
marked de<br />
bosentan,<br />
uses of<br />
all<br />
In placebo-controlled studies of<br />
ent.<br />
bosentan-treated<br />
6% of<br />
in<br />
observed<br />
were<br />
g/dL)<br />
11<br />
<<br />
values<br />
in<br />
ng<br />
25<br />
and<br />
125<br />
of<br />
doses<br />
with<br />
treated<br />
hypertension<br />
arterial<br />
pulmonary<br />
h<br />
hemoglo<br />
in<br />
A decrease<br />
patients.<br />
placebo-treated<br />
1% in<br />
to<br />
compared<br />
placebo-treated pat<br />
bosentan-treated patients as compared to 29% of<br />
b<br />
of<br />
6 weeks<br />
the decrease occurred during the first<br />
1 g/dL,<br />
least<br />
at<br />
y<br />
bosent<br />
68% of<br />
in<br />
limits<br />
normal<br />
within<br />
remained<br />
concentration<br />
obin<br />
does<br />
it<br />
but<br />
known,<br />
not<br />
the change in hemoglobin is<br />
e explanation for<br />
a<br />
1 and 3 months,<br />
be checked after<br />
hemoglobin concentrations<br />
d that<br />
undertaken<br />
be<br />
should<br />
evaluation<br />
further<br />
occurs,<br />
concentration<br />
lobin<br />
on:<br />
etentio<br />
re<br />
d<br />
id<br />
h<br />
c<br />
severe<br />
with<br />
patients<br />
of<br />
trial<br />
placebo-controlled<br />
a<br />
n<br />
I<br />
Aminotransferase elevations<br />
n.<br />
be measured prior<br />
levels must<br />
must<br />
monitoring and treatment<br />
vomiting,<br />
nausea,<br />
as<br />
(such<br />
ury<br />
stopped.<br />
be<br />
should<br />
treatment<br />
ment:<br />
rm<br />
mpair<br />
Im<br />
er<br />
ive<br />
L<br />
o<br />
amin<br />
iver<br />
L -<br />
be avoided in<br />
should generally<br />
with elevated<br />
in patients<br />
ided<br />
see<br />
( G<br />
ARNIN<br />
OX WA<br />
B .<br />
)<br />
hemato<br />
and<br />
hemoglobin<br />
in<br />
e<br />
s -<br />
mean decrease<br />
The overall<br />
hs.<br />
of<br />
decrease<br />
this<br />
of<br />
Most<br />
ent).<br />
by<br />
stabilized<br />
levels<br />
emoglobin<br />
creases in hemoglobin (> 15%<br />
placebo-<br />
3% of<br />
and<br />
patients<br />
decreases<br />
marked<br />
b.i.d.,<br />
mg<br />
0<br />
least<br />
at<br />
by<br />
concentration<br />
obin<br />
those patients<br />
In 80% of<br />
ients.<br />
During the<br />
osentan treatment.<br />
compared<br />
patients<br />
an-treated<br />
to be hemorrhage<br />
appear<br />
not<br />
thereafter.<br />
3 months<br />
and every<br />
and<br />
cause<br />
the<br />
determine<br />
to<br />
n<br />
was<br />
there<br />
failure<br />
heart<br />
hronic<br />
been establish<br />
patients have not<br />
of<br />
number<br />
included a sufficient<br />
IONS:<br />
DVERSE REACTI<br />
A<br />
e<br />
ve<br />
Adv<br />
a<br />
hemoglobin and hematocrit<br />
of<br />
patien<br />
777<br />
in<br />
open-label)<br />
4<br />
and<br />
(125<br />
dose<br />
clinical<br />
recommended<br />
yea<br />
4.1<br />
to<br />
day<br />
1<br />
from<br />
ranged<br />
hypertension<br />
arterial<br />
pulmonary<br />
Tr<br />
N = 28 more than 12 months).<br />
trials in patien<br />
during the clinical<br />
2/80 patients).<br />
on placebo (3%;<br />
The adv<br />
function.<br />
liver<br />
abnormal<br />
on bosentan in placebo-controll<br />
able 1.<br />
Ta<br />
r<br />
Adverse events* occu<br />
placebo-controlled studies in pu<br />
Adverse Event<br />
Headache<br />
Nasopharyngitis<br />
Flushing<br />
Hepatic function abnormal<br />
limb<br />
lower<br />
dema,<br />
E<br />
ed.<br />
h :<br />
ents<br />
atie<br />
y Pa<br />
erly<br />
de<br />
n Eld<br />
se in<br />
Us<br />
R<br />
experience with TRACLEE<br />
linical<br />
C<br />
u<br />
in s<br />
jects to identify a difference in response between elderly an<br />
such subj<br />
s:<br />
ents<br />
Eve<br />
se<br />
rs<br />
e<br />
e<br />
S<br />
G<br />
ARNIN<br />
OX WA<br />
B<br />
d<br />
an<br />
injury<br />
liver<br />
of<br />
discussion<br />
or<br />
f<br />
P<br />
Safety data on bosentan were obtained from 12 clinica<br />
abnormalities.<br />
Dose<br />
diseases.<br />
other<br />
and<br />
hypertension,<br />
arterial<br />
pulmonary<br />
with<br />
nts<br />
exposu<br />
The<br />
durations.<br />
of<br />
variety<br />
a<br />
for<br />
administered<br />
were<br />
b.i.d.)<br />
mg<br />
5<br />
mor<br />
for<br />
39<br />
=<br />
N<br />
and<br />
years<br />
1.5<br />
for<br />
61<br />
=<br />
N<br />
year;<br />
1<br />
for<br />
89<br />
=<br />
(N<br />
rs<br />
(N =<br />
years<br />
1.7<br />
to<br />
day<br />
from 1<br />
ranged<br />
bosentan<br />
to<br />
235)<br />
(N =<br />
patients<br />
n<br />
than those rela<br />
discontinuations due to adverse events other<br />
eatment<br />
on bose<br />
hypertension were more frequent<br />
nts with pulmonary arterial<br />
and occurrin<br />
discontinuations >1%,<br />
In this database the only cause of<br />
the bosentan-treated pa<br />
occurred in ≥ 3% of<br />
verse drug reactions that<br />
250 mg<br />
125 or<br />
doses of<br />
hypertension at<br />
ed trials in pulmonary arterial<br />
and<br />
patients treated with bosentan 125-250 mg b.i.d.<br />
ring in ≥ 3% of<br />
hypertension<br />
ulmonary arterial<br />
osentan (N = 165)<br />
B<br />
l<br />
P<br />
o.<br />
N % .<br />
<strong>No</strong><br />
6<br />
3 %<br />
2<br />
2 6<br />
1<br />
8<br />
1 %<br />
1<br />
1 6<br />
5<br />
1 %<br />
9 4<br />
4<br />
1 %<br />
8 2<br />
3<br />
1 %<br />
8 4<br />
has not<br />
older<br />
jects aged 65 or<br />
bj<br />
patients.<br />
d younger<br />
RECAUTIONS<br />
P<br />
n<br />
discussio<br />
for<br />
studies (8 placebo-controlled<br />
al<br />
currently<br />
the<br />
times<br />
8<br />
to<br />
up<br />
es<br />
trials<br />
these<br />
in<br />
bosentan<br />
to<br />
ure<br />
of<br />
Exposure<br />
years).<br />
2<br />
than<br />
re<br />
and<br />
months<br />
6<br />
than<br />
more<br />
126<br />
=<br />
ated to pulmonary hypertension<br />
than<br />
8/165 patients)<br />
ntan (5%;<br />
g more often on bosentan was<br />
tients and were more common<br />
are shown in Table 1:<br />
g b.i.d.<br />
more common on bosentan in<br />
lacebo (N = 80)<br />
%<br />
20%<br />
8%<br />
5%<br />
3%<br />
5%<br />
treatment.<br />
specific<br />
for<br />
eed<br />
n<br />
u<br />
lu<br />
Fl<br />
hospit<br />
an increased incidence of<br />
TRACLEER<br />
with<br />
treatment<br />
f<br />
o ® .<br />
occurr<br />
hypertension,<br />
pulmonary<br />
management, or hospitalization<br />
of pulmonary edema occur when<br />
TRACLEER ®<br />
discontinued.<br />
should be<br />
trial experience with the use of<br />
with antiretroviral medications.<br />
increased plasma concentrations<br />
Interactions<br />
potential<br />
). Due to the<br />
the efficacy of some antiretroviral<br />
due to the inhibition of organic<br />
bosentan. The potential for an increased risk of hepatic toxicity and hematological adverse events cannot be excluded.<br />
s:<br />
Patients<br />
nformation for<br />
I<br />
i<br />
Pat<br />
th<br />
with<br />
discuss<br />
should<br />
physician<br />
avoida<br />
of<br />
and<br />
testing<br />
pregnancy<br />
their<br />
with<br />
pregnancy<br />
prevent<br />
to<br />
as needed.<br />
sought<br />
rug Interactions:<br />
D<br />
s<br />
Bosentan i<br />
bosentan (see ketocon<br />
tration of<br />
(such as<br />
and a CYP3A4 inhibitor<br />
Co<br />
bosentan.<br />
of<br />
concentrations<br />
RACLEER<br />
T ® d<br />
t<br />
i<br />
on:<br />
etentio<br />
re<br />
d<br />
id<br />
h<br />
c<br />
severe<br />
with<br />
patients<br />
of<br />
trial<br />
controlled<br />
placebo<br />
a<br />
In<br />
gain and increased leg ede<br />
associated with weight<br />
CHF<br />
talization for<br />
fl<br />
of<br />
reports<br />
post-marketing<br />
numerous<br />
been<br />
have<br />
there<br />
addition,<br />
In<br />
TRACLEER<br />
starting<br />
after<br />
weeks<br />
within<br />
ng<br />
i ® intervention<br />
required<br />
Patients<br />
.<br />
for decompensating heart failure.<br />
hospitalization<br />
Disease<br />
eno-Occlusive<br />
Pulmonary Veno-Occlusive<br />
TRACLEER<br />
when ® PVOD<br />
is administered the possibility of associated<br />
discontinued.<br />
Infection:<br />
Pulmonary Arterial Hypertension Associated with HIV<br />
TRACLEER<br />
of ® who<br />
AH associated with HIV infection<br />
in patients with PAH<br />
medications. An interaction study between bosentan and lopinavir+ritonavir<br />
concentrations of bosentan and decreased concentrations of lopinavir+ritonavir<br />
potential for interactions related to the inducing effect of bosentan on<br />
antiretroviral therapies, patients should be monitored carefully regarding their<br />
(OATP) by ritonavir, there may be<br />
organic anion-transporting polypeptides (OATP)<br />
bosentan. The potential for an increased risk of hepatic toxicity and hematological adverse events cannot be excluded.<br />
the TRACLEER<br />
are advised to consult<br />
nts<br />
e ® e<br />
Medication Guide on th<br />
serum aminotra<br />
of<br />
monitoring<br />
monthly<br />
of<br />
importance<br />
the<br />
patient<br />
he<br />
effective<br />
for<br />
options<br />
discuss<br />
should<br />
physician<br />
The<br />
pregnancy.<br />
of<br />
ance<br />
adeq<br />
on<br />
expert<br />
similar<br />
or<br />
gynecologist<br />
from a<br />
Input<br />
patients.<br />
female<br />
these enzymes ma<br />
Inhibition of<br />
s metabolized by CYP2C9 and CYP3A4.<br />
(such<br />
both a CYP2C9 inhibitor<br />
administration of<br />
Concomitant<br />
nazole).<br />
lea<br />
likely<br />
with bosentan will<br />
ritonavir)<br />
or<br />
itraconazole,<br />
ketoconazole,<br />
inhibitor<br />
CYP2C9<br />
potent<br />
a<br />
of<br />
combinations<br />
such<br />
of<br />
-administration<br />
l<br />
tl<br />
C<br />
CYP2C9<br />
d<br />
CYP3A4<br />
f<br />
d<br />
i<br />
i<br />
t<br />
d B<br />
was<br />
there<br />
failure,<br />
heart<br />
hronic<br />
4-8 weeks<br />
ema during the first<br />
with<br />
patients<br />
in<br />
retention<br />
uid<br />
intervention with a diuretic, fluid<br />
(PVOD):<br />
Disease<br />
signs<br />
Should<br />
PVOD should be considered and<br />
Infection:<br />
clinical<br />
There is limited<br />
who are treated concomitantly<br />
lopinavir+ritonavir in healthy subjects showed<br />
(see<br />
lopinavir+ritonavir<br />
Drug<br />
PRECAUTIONS:<br />
on CYP450, which could affect<br />
their HIV infection. Conversely,<br />
be an increase in exposure to<br />
bosentan. The potential for an increased risk of hepatic toxicity and hematological adverse events cannot be excluded.<br />
TRACLEER<br />
safe use of<br />
e ® e<br />
Th<br />
.<br />
serum<br />
or<br />
urine<br />
and<br />
ansferases<br />
measures<br />
and<br />
contraception<br />
e<br />
be<br />
should<br />
contraception<br />
quate<br />
increase the plasma concen<br />
y -<br />
amiodarone)<br />
fluconazole or<br />
as<br />
in plasma<br />
d to large increases<br />
with<br />
inhibitor<br />
CYP3A4<br />
a<br />
plus<br />
d<br />
f<br />
ti<br />
t<br />
,<br />
Hypotension<br />
Palpitations<br />
Dyspepsia<br />
Edema<br />
Fatigue<br />
Pruritus<br />
ons<br />
with<br />
AEs<br />
only<br />
*<strong>No</strong>te:<br />
3<br />
least<br />
s (at<br />
reported events<br />
associated with the use o<br />
common in the treated pop<br />
In placebo-controlled studies of<br />
failure), a total of 677 patients<br />
were treated with placebo. The<br />
occurred in ≥ 3% of bosentan-treated<br />
(≥ 2% difference) were headache<br />
(5% vs. 1%), and anemia (3% vs. 1%).<br />
Experience<br />
Post-Marketing<br />
post-marketing rep<br />
been several<br />
ho<br />
8<br />
of<br />
range<br />
a<br />
within<br />
occurred<br />
w<br />
resolved<br />
angioedema<br />
of<br />
signs<br />
unexplained hepat<br />
of<br />
are cases<br />
r<br />
1<br />
1 %<br />
7 3<br />
8 %<br />
5 1<br />
7 %<br />
4 0<br />
7 %<br />
4 2<br />
6 %<br />
4 1<br />
6 %<br />
4 0<br />
tre<br />
of<br />
end<br />
after<br />
day<br />
calendar<br />
1<br />
to<br />
treatment<br />
of<br />
from start<br />
et<br />
a<br />
to be informative,<br />
those too general<br />
are included except<br />
3%)<br />
were associated with the condition<br />
the drug because they<br />
f<br />
pulation.<br />
of bosentan in pulmonary arterial hypertension and for other diseases<br />
patients were treated with bosentan at daily doses ranging from 100 mg<br />
The duration of treatment ranged from 4 weeks to 6 months. For the<br />
bosentan-treated patients, the only ones that occurred more frequently on<br />
headache (16% vs. 13%), flushing (7% vs. 2%), abnormal hepatic function<br />
(5% vs. 1%), and anemia (3% vs. 1%).<br />
: u<br />
req<br />
Anemia<br />
Jaundice,<br />
Thrombocytopenia,<br />
Rash,<br />
Hypersensitivity,<br />
T<br />
bosentan.<br />
edema associated with the use of<br />
angioneurotic<br />
of<br />
ports<br />
w<br />
treated<br />
were<br />
patients<br />
Some<br />
therapy.<br />
starting<br />
after<br />
days<br />
21<br />
to<br />
ours<br />
TRACLEER<br />
discontinuing<br />
ithout<br />
w ® h<br />
t<br />
in<br />
period,<br />
post-marketing<br />
the<br />
In<br />
.<br />
wit<br />
therapy<br />
prolonged (> 12 months)<br />
were reported after<br />
cirrhosis<br />
ic<br />
t<br />
4%<br />
1%<br />
0%<br />
3%<br />
1%<br />
0%<br />
All<br />
included.<br />
are<br />
eatment<br />
reasonably<br />
and those not<br />
are very<br />
being treated or<br />
diseases (primarily chronic heart<br />
to 2000 mg and 288 patients<br />
the adverse drug reactions that<br />
on bosentan than on placebo<br />
function (6% vs. 2%), leg edema<br />
have<br />
There<br />
transfusion:<br />
uiring<br />
the reported cases<br />
of<br />
he onset<br />
their<br />
and<br />
antihistamine<br />
an<br />
with<br />
monitoring,<br />
close<br />
of<br />
setting<br />
he<br />
h TRACLEER<br />
t ® h<br />
wit<br />
n patients<br />
i<br />
RACLEER<br />
T ® e<br />
recommend<br />
not<br />
is<br />
isozy<br />
two<br />
these<br />
by<br />
metabolized<br />
vi<br />
in<br />
isozyme<br />
CYP<br />
any<br />
on<br />
effect<br />
increase the plasma concentrations of drugs metabolized by these enzymes.<br />
Hormonal Contraceptives, Including<br />
tion study demonstrated that<br />
norethindrone<br />
of<br />
decreases<br />
average<br />
56<br />
as<br />
much<br />
as<br />
were<br />
exposure<br />
transdermal,<br />
injectable,<br />
oral,<br />
should practice additional methods<br />
Specific interaction studies have demonstrated the following:<br />
yclosporine A:<br />
C<br />
r<br />
fi<br />
the<br />
During<br />
bosentan<br />
Steady-state<br />
30-fold.<br />
b<br />
administration of<br />
oncomitant<br />
c<br />
pl<br />
Consequently<br />
CYP2C9.<br />
and<br />
CYP3A4<br />
of<br />
inducer<br />
an<br />
is<br />
Bosentan<br />
ed.<br />
TRACLEER<br />
when<br />
decreased<br />
be<br />
will<br />
es<br />
m ® n<br />
Bose<br />
co-administered.<br />
is<br />
T<br />
Consequently,<br />
CYP3A4).<br />
CYP2D6,<br />
CYP2C19,<br />
CYP2C9,<br />
(CYP1A2,<br />
tro<br />
increase the plasma concentrations of drugs metabolized by these enzymes.<br />
ransdermal, and Implantable Contraceptives:<br />
Including Oral, Injectable, Transdermal,<br />
co-administration of bosentan and the oral hormonal contraceptive<br />
respectively<br />
31%,<br />
and<br />
14%<br />
of<br />
levels<br />
estradiol<br />
ethinyl<br />
and<br />
norethindrone<br />
hormo<br />
Therefore,<br />
jects.<br />
subj<br />
individual<br />
in<br />
respectively,<br />
66%,<br />
% and<br />
TRACLEER<br />
when<br />
reliable<br />
be<br />
not<br />
may<br />
forms,<br />
implantable<br />
and ®<br />
methods of contraception and not rely on hormonal contraception alone<br />
Specific interaction studies have demonstrated the following:<br />
bose<br />
of<br />
concentrations<br />
trough<br />
administration,<br />
concomitant<br />
of<br />
day<br />
st<br />
abs<br />
the<br />
in<br />
than<br />
higher<br />
4-fold<br />
to<br />
3-<br />
were<br />
concentrations<br />
plasma<br />
sentan and cyclosporine A is contraindicated (see<br />
o<br />
A<br />
RAINDIC<br />
ONTR<br />
C<br />
drugs<br />
of<br />
concentrations<br />
asma<br />
inhibitory<br />
relevant<br />
no<br />
had<br />
tan<br />
RACLEER<br />
T ® o<br />
t<br />
expected<br />
not<br />
is<br />
Contraceptives:<br />
interac<br />
An -<br />
Ortho-<strong>No</strong>vum<br />
contraceptive<br />
® produced<br />
in<br />
decreases<br />
However,<br />
respectively.<br />
including<br />
contraceptives,<br />
onal<br />
Women<br />
co-administered.<br />
is<br />
when taking TRACLEER<br />
alone ® .<br />
about<br />
by<br />
increased<br />
were<br />
ntan<br />
The<br />
A.<br />
cyclosporine<br />
of<br />
sence<br />
TIONS<br />
AT<br />
f<br />
Co-administration o<br />
).<br />
p<br />
p<br />
multiple co-morbidities and drug<br />
be excluded (se<br />
cases could not<br />
Manufactured by:<br />
Inc.<br />
Patheon,<br />
L5N 7K9,<br />
Ontario,<br />
Mississauga,<br />
previous pa<br />
References for<br />
pulmona<br />
Bosentan therapy for<br />
the d<br />
Effects of<br />
al.<br />
et<br />
Sitbon O,<br />
a randomised placebo-control<br />
© 2009 Actelion Pharmaceutica<br />
py<br />
)<br />
(<br />
g<br />
p<br />
p<br />
The contr<br />
failure.<br />
liver<br />
There have also been rare reports of<br />
g therapies.<br />
e<br />
G<br />
ARNIN<br />
BOX WA<br />
Marketed by:<br />
).<br />
Inc.<br />
Actelion Pharmaceuticals US,<br />
USA<br />
CA 94080,<br />
South San Francisco,<br />
CANADA<br />
ges:<br />
a .<br />
1 .<br />
Actelion Pharmaceuticals<br />
ata on file,<br />
D .<br />
2 s<br />
Bade<br />
Rubin LJ,<br />
hypertension.<br />
y arterial<br />
r d.<br />
J Me<br />
ngl<br />
En<br />
N .<br />
2002;346:896-903<br />
d .<br />
3 a<br />
Ch<br />
bosentan in patients with pul<br />
antagonist<br />
endothelin-receptor<br />
dual<br />
ed study.<br />
l t.<br />
ance<br />
L .<br />
2001;358:1119-1123<br />
t<br />
07 354 01 03 0209<br />
rights reserved.<br />
All<br />
Inc.<br />
ls US,<br />
a<br />
p<br />
TRACLEER<br />
ibution of<br />
r ® e<br />
in thes<br />
al.<br />
et<br />
RJ,<br />
Barst<br />
sch DB,<br />
Simonneau G,<br />
annick RN,<br />
monary hypertension:<br />
1200 Folsom Street San Francisco, CA 94103 tel 415.421.2900 fax 415.421.5842 www.andresendigital.com
We can’t thank you enough.<br />
Take a closer look and see how much you helped raise<br />
at the Gilead Sciences event during CHEST 2008, for the<br />
benefit of the Pulmonary Hypertension Association.<br />
Physicians from around the world joined in this effort<br />
Argentina, Austria, Brazil, Canada, Colombia, Egypt, Greece, India, Ireland, Italy, Japan,<br />
Kuwait, Malaysia, Mexico, Nigeria, Portugal, Romania, Saudi Arabia, Serbia, South Korea,<br />
Switzerland, Turkey, United States of America<br />
Proudly sponsored by<br />
For the benefit of<br />
© 2008 Gilead Sciences, Inc. All rights reserved. ABS3187 December 2008<br />
Gilead and the Gilead logo are trademarks of Gilead Sciences, Inc.
Program Announcement:<br />
New Application Deadline: February 12, 2009 Resubmission Deadline: March 12, 2009<br />
New Application Deadline: June 12, 2009 Resubmission Deadline: July 12, 2009<br />
Pulmonary Hypertension<br />
Association (<strong>PHA</strong>)<br />
National Heart, Lung, and<br />
Blood Institute (NHLBI)<br />
Jointly Sponsored<br />
Mentored Clinical Scientist Development Award (K08) &<br />
Mentored Patient-Oriented Research Career Development Award (K23)<br />
PURPOSE: K08<br />
• To support the development of outstanding clinician research<br />
scientists in the area of pulmonary hypertension.<br />
• To provide specialized study for clinically trained professionals<br />
who are committed to a career in research in pulmonary<br />
hypertension and have the potential to develop into independent<br />
investigators.<br />
• To support a 3 to 5 year period of supervised research experience<br />
that integrates didactic studies with laboratory or clinically<br />
based research.<br />
• To support research that has both intrinsic research importance<br />
and merit as a vehicle for learning the methodology, theories,<br />
and conceptualizations necessary for a well-trained independent<br />
researcher.<br />
MECHANISM:<br />
Awards in response to the program announcement will use the<br />
National Institutes of Health (NIH) K08 or the K23 mechanism.<br />
PURPOSE: K23<br />
• To support career development of investigators who have<br />
made a commitment to focus their research endeavors on<br />
patient-oriented research.<br />
• To support a 3 to 5 year period of supervised study and<br />
research for clinically trained professionals who have the<br />
potential to develop into productive, clinical investigators<br />
focusing on patient-oriented research in pulmonary hypertension.<br />
• To support patient-oriented research, which is defined as<br />
research conducted with human subjects (or on material of<br />
human origin, such as tissues, specimens, and cognitive<br />
phenomena) for which an investigator directly interacts with<br />
human subjects.<br />
• To support areas of research that include: 1) mechanisms of<br />
human disease; 2) therapeutic interventions; 3) clinical trials;<br />
and 4) development of new technologies.<br />
FUNDING:*<br />
The award will be funded by <strong>PHA</strong> and NHLBI and the KO8<br />
and/or the K23 will be awarded in 2009.<br />
FOR MORE INFORMATION:<br />
Visit: www.<strong>PHA</strong>ssociation.org/support/ResearchFunding.asp<br />
* Restrictions apply. Please see complete announcement<br />
at the website listed above.<br />
Advances in<br />
Pulmonary Hypertension<br />
Pulmonary Hypertension Association<br />
801 Roeder Road, Suite 400<br />
Silver Spring, MD 20910-4496<br />
<strong>No</strong>n-Profit Org.<br />
US POSTAGE<br />
PAID<br />
Tampa, FL<br />
Permit #995<br />
To order additional copies, call or contact <strong>PHA</strong> at 1-866-474-4742 or www.<strong>PHA</strong>ssociation.org.<br />
All issues of Advances in Pulmonary Hypertension are also available online at www.<strong>PHA</strong>ssociation.org/Medical/Advances_in_PH