Advances in Pulmonary Hypertension - PHA Online University
Advances in Pulmonary Hypertension - PHA Online University
Advances in Pulmonary Hypertension - PHA Online University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Advances</strong> <strong>in</strong><br />
<strong>Pulmonary</strong><br />
<strong>Hypertension</strong><br />
Official Journal of the <strong>Pulmonary</strong> <strong>Hypertension</strong> Association<br />
Autumn 2002<br />
Vol 1, No 2<br />
Special Feature:<br />
Pull-Out Cl<strong>in</strong>ical Algorithm
Editorial Mission<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> is committed<br />
to help physicians <strong>in</strong> their cl<strong>in</strong>ical decision mak<strong>in</strong>g by<br />
<strong>in</strong>form<strong>in</strong>g them of important trends affect<strong>in</strong>g their<br />
practice. Analyz<strong>in</strong>g the impact of new f<strong>in</strong>d<strong>in</strong>gs and<br />
cover<strong>in</strong>g current <strong>in</strong>formation <strong>in</strong> the peer-reviewed literature,<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> is published<br />
four times a year. <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
is the official journal of the <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
Association.<br />
Each article <strong>in</strong> this journal has been reviewed and<br />
approved by members of the Editorial Advisory Board.<br />
Editorial Advisory Board<br />
Editor-<strong>in</strong>-Chief<br />
Victor F. Tapson, MD<br />
Associate Professor of Medic<strong>in</strong>e<br />
Division of <strong>Pulmonary</strong> and Critical Care Medic<strong>in</strong>e<br />
Duke <strong>University</strong> Medical Center<br />
Durham, North Carol<strong>in</strong>a<br />
Sean Ga<strong>in</strong>e, MD, PhD<br />
Director, <strong>Pulmonary</strong> <strong>Hypertension</strong> Unit<br />
Mater Misericordiae Hospital<br />
<strong>University</strong> College<br />
Dubl<strong>in</strong>, Ireland<br />
Vallerie McLaughl<strong>in</strong>, MD<br />
Assistant Professor of Medic<strong>in</strong>e<br />
Associate Director<br />
Center for <strong>Pulmonary</strong> Heart Disease<br />
Coronary Heart Disease Detection<br />
and Treatment Center<br />
Rush Heart Institute<br />
Rush Presbyterian-St. Luke’s Medical Center<br />
Chicago, Ill<strong>in</strong>ois<br />
Ivan M. Robb<strong>in</strong>s, MD<br />
Director, <strong>Pulmonary</strong> <strong>Hypertension</strong> Center<br />
Allergy/<strong>Pulmonary</strong> and Critical Care Medic<strong>in</strong>e<br />
Vanderbilt <strong>University</strong><br />
Nashville, Tennessee<br />
Publisher<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Association<br />
Bruce Brundage, MD, President<br />
R<strong>in</strong>o Aldrighetti, Executive Director<br />
Publish<strong>in</strong>g Staff<br />
Stu Chapman, Executive Editor<br />
Susan Chapman, Manag<strong>in</strong>g Editor<br />
Heidi Green, Associate Editor<br />
Gloria Catalano, Production Director<br />
Michael McCla<strong>in</strong>, Design Director<br />
<strong>PHA</strong> Office<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Association<br />
850 Sligo Avenue, Suite 800<br />
Silver Spr<strong>in</strong>g, MD 20910<br />
301-565-3004, 301-565-3994 (fax)<br />
www.phassociation.org<br />
Provided through an unrestricted educational grant<br />
from Actelion Pharmaceuticals, U.S., Inc. and<br />
Accredo Therapeutics.<br />
© 2002 by <strong>Pulmonary</strong> <strong>Hypertension</strong> Association and<br />
DataMedica. All rights reserved. None of the contents<br />
may be reproduced <strong>in</strong> any form whatsoever without the<br />
written permission of the executive editor.<br />
Editorial Offices<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong>, DataMedica,<br />
424 Dune Road, Westhampton Beach, NY 11978<br />
Tel (631) 288-4702<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> is circulated<br />
to cardiologists, pulmonologists, rheumatologists<br />
and other selected physicians by the <strong>Pulmonary</strong><br />
<strong>Hypertension</strong> Association. The contents are<br />
<strong>in</strong>dependently determ<strong>in</strong>ed by the Editor and the<br />
Editorial Advisory Board.<br />
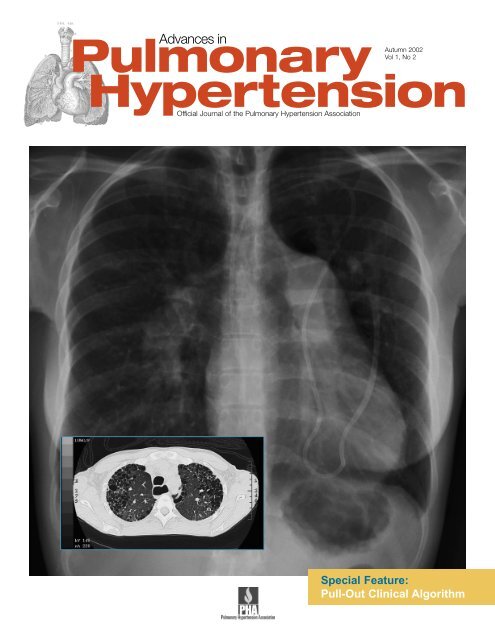
Cover Photo: Chest x-ray depicts pulmonary hypertension and a<br />
Hickman catheter. In lower left, computed tomography shows<br />
<strong>in</strong>terstitial lung disease and the dilated esophagus from<br />
scleroderma. (Courtesy of Victor F. Tapson, MD)<br />
Pr<strong>in</strong>ted on recycled paper.<br />
The Scientific Leadership<br />
Council of the <strong>Pulmonary</strong><br />
<strong>Hypertension</strong> Association<br />
The scientific program of the <strong>Pulmonary</strong><br />
<strong>Hypertension</strong> Association is guided by the<br />
association’s Scientific Leadership Council.<br />
The Council <strong>in</strong>cludes the follow<strong>in</strong>g health<br />
care professionals:<br />
Michael McGoon, MD<br />
SLC Chair<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong><br />
Cl<strong>in</strong>ic/Mayo Cl<strong>in</strong>ic<br />
Rochester, M<strong>in</strong>nesota<br />
Robyn Barst, MD<br />
SLC Vice-Chair<br />
Columbia Presbyterian Medical Center<br />
Babies Hospital<br />
New York, New York<br />
David B. Badesch, MD<br />
Chair, SLC Nom<strong>in</strong>ations Committee<br />
<strong>University</strong> of Colorado<br />
Health Sciences Center<br />
Denver, Colorado<br />
Joy Beckmann, RN, MSN<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Program<br />
Harbor-UCLA Medical Center<br />
Torrance, California<br />
C. Gregory Elliott, MD<br />
Chair, SLC Publications Committee<br />
LDS Hospital<br />
<strong>University</strong> of Utah<br />
School of Medic<strong>in</strong>e<br />
Salt Lake City, Utah<br />
Adaani Frost, MD<br />
Baylor College of Medic<strong>in</strong>e<br />
Houston, Texas<br />
Sean Ga<strong>in</strong>e, MD, PhD<br />
Mater Misericordiae Hospital<br />
Dubl<strong>in</strong>, Ireland<br />
Nazzareno Galiè, MD<br />
Istituto di Malattie dell’ Apparato<br />
Cardiovascolare<br />
Universita di Bologna<br />
Bologna, Italy<br />
Abby Krichman, RRT<br />
Division of <strong>Pulmonary</strong> and<br />
Critical Care Medic<strong>in</strong>e<br />
Duke <strong>University</strong> Medical Center<br />
Durham, North Carol<strong>in</strong>a<br />
David Langleben, MD<br />
Jewish General Hospital<br />
Montreal, Quebec, Canada<br />
James E. Loyd, MD<br />
<strong>Pulmonary</strong> and Critical Care Medic<strong>in</strong>e<br />
Vanderbilt <strong>University</strong> Medical Center<br />
Nashville, Tennessee<br />
Vallerie McLaughl<strong>in</strong>, MD<br />
Rush Presbyterian-St. Lukes<br />
Medical Center<br />
Chicago, Ill<strong>in</strong>ois<br />
John H. Newman, MD<br />
Nashville VA Hospital<br />
Nashville, Tennessee<br />
Horst Olschewski, MD<br />
Justus-Liebig Universitat<br />
Gessen, Germany<br />
Harold I. Palevsky, MD<br />
<strong>University</strong> of Pennsylvania<br />
School of Medic<strong>in</strong>e<br />
Presbyterian Medical Center<br />
Philadelphia, Pennsylvania<br />
Stuart Rich, MD<br />
Vice-Chair, SLC Research Committee<br />
Rush Heart Institute-<br />
Center for <strong>Pulmonary</strong> Heart Disease<br />
Chicago, Ill<strong>in</strong>ois<br />
Ivan M. Robb<strong>in</strong>s, MD<br />
Chair, SLC Consensus Committee<br />
Vanderbilt <strong>University</strong><br />
Nashville, Tennessee<br />
Lewis J. Rub<strong>in</strong>, MD<br />
Chair, SLC Research Committee<br />
<strong>University</strong> of California at San Diego<br />
San Diego, California<br />
Cathy J. Severson, RN, BSN<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong><br />
Cl<strong>in</strong>ic/Mayo Cl<strong>in</strong>ic<br />
Rochester, M<strong>in</strong>nesota<br />
Victor F. Tapson, MD<br />
Division of <strong>Pulmonary</strong> and Critical<br />
Care Medic<strong>in</strong>e<br />
Duke <strong>University</strong> Medical Center<br />
Durham, North Carol<strong>in</strong>a<br />
Allison Widlitz, PA<br />
Pediatric Cardiology<br />
New York Presbyterian Hospital<br />
New York, New York<br />
Carol E. Vreim, PhD NHLBI Liaison<br />
Deputy Director<br />
Division of Lung Diseases, NHBLI<br />
Bethesda, Maryland<br />
Emeritus<br />
Bruce H. Brundage, MD<br />
Heart Institute of the Cascades<br />
Bend, Oregon<br />
Alfred P. Fishman, MD<br />
<strong>University</strong> of Pennsylvania<br />
Health System<br />
Philadelphia, Pennsylvania
Editor’s Memo<br />
Bridg<strong>in</strong>g the Gap Between<br />
Specialists With Insights<br />
From an Expert Panel<br />
Often unsuspected and underappreciated,<br />
pulmonary arterial hypertension <strong>in</strong> some<br />
forms of scleroderma is much like primary<br />
pulmonary hypertension; the cause of the<br />
dyspnea and associated symptoms frequently<br />
eludes diagnosis until very late <strong>in</strong> the disease.<br />
Unfortunately, by that time, pulmonary<br />
hypertension may have reached an advanced<br />
stage less amenable to treatment. Although many patients with<br />
collagen vascular disease are considered at high risk for <strong>in</strong>flammatory<br />
pulmonary <strong>in</strong>volvement, the disease process is often<br />
<strong>in</strong>sidious and appropriate evaluation, <strong>in</strong>clud<strong>in</strong>g pulmonary<br />
function tests, echocardiography, and right-heart catheterization,<br />
may not be ordered when the benefit of treatment might<br />
be more substantial. One of the key issues concerns separat<strong>in</strong>g<br />
the potential causes of pulmonary hypertension that may occur<br />
<strong>in</strong> these patients and recogniz<strong>in</strong>g why they may require more<br />
aggressive follow-up to identify an evolv<strong>in</strong>g <strong>in</strong>flammatory component<br />
and potential progressive vasculopathy.<br />
Driven to F<strong>in</strong>d Cure<br />
for PH, Brundage<br />
Leads New Quest for<br />
Breakthrough <strong>in</strong> Therapy<br />
Bruce Brundage, MD, has seen it all,<br />
from the first anecdotal cases when<br />
no treatment for pulmonary hypertension<br />
was available, to the landmark<br />
National Institutes of Health<br />
(NIH) registry, to the large scale<br />
cl<strong>in</strong>ical trials of today <strong>in</strong>volv<strong>in</strong>g hundreds<br />
of patients. For anyone seek<strong>in</strong>g<br />
to chart progress <strong>in</strong> the field, his<br />
career serves as a bridge, spann<strong>in</strong>g<br />
milestones <strong>in</strong> the treatment of the disease. He has been<br />
associated with virtually every key development <strong>in</strong> the<br />
progress toward a cure, beg<strong>in</strong>n<strong>in</strong>g <strong>in</strong> the 1970s when he<br />
served as the director of the cardiac catheterization laboratory<br />
at the <strong>University</strong> of California, San Francisco.<br />
It was dur<strong>in</strong>g his study of those early cases, when he<br />
<strong>in</strong>serted catheters <strong>in</strong>to the pulmonary artery to measure<br />
the effects of various vasodilat<strong>in</strong>g drugs, that he began<br />
focus<strong>in</strong>g on pulmonary hypertension. Dur<strong>in</strong>g the last 25<br />
For these reasons, the <strong>Pulmonary</strong> <strong>Hypertension</strong> Roundtable<br />
discussion <strong>in</strong> this issue will have broad appeal to our<br />
readership, which <strong>in</strong>cludes cardiologists, pulmonologists, and<br />
rheumatologists. Clearly, this is a disease that is likely to present<br />
to each of these groups, and by transcend<strong>in</strong>g the boundaries<br />
of these specialties, our discussion offers <strong>in</strong>sights about<br />
its <strong>in</strong>cidence, evolution, and appropriate evaluation. From the<br />
diverse backgrounds of our experts, you will f<strong>in</strong>d cl<strong>in</strong>ical<br />
pearls relevant to your practice regardless of your specialty.<br />
One of the goals of <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> is to<br />
br<strong>in</strong>g you this k<strong>in</strong>d of distilled knowledge, based on contributions<br />
from the Scientific Leadership Council of the <strong>Pulmonary</strong><br />
<strong>Hypertension</strong> Association (<strong>PHA</strong>). These physicians are listed<br />
on page 2 and provide a tremendous breadth of experience<br />
gathered at lead<strong>in</strong>g <strong>in</strong>stitutions throughout the world. Their<br />
cont<strong>in</strong>ued <strong>in</strong>volvement with the journal gives us one of the<br />
clearest perspectives on the latest <strong>in</strong>formation <strong>in</strong> diagnosis<br />
and treatment.<br />
We appreciate the warm welcome <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong><br />
<strong>Hypertension</strong> has received <strong>in</strong> the medical community and<br />
look forward to fulfill<strong>in</strong>g the commitment <strong>PHA</strong> has made<br />
toward expand<strong>in</strong>g your awareness of manag<strong>in</strong>g this disease.<br />
We also welcome your comments and suggestions regard<strong>in</strong>g<br />
articles that appear <strong>in</strong> this issue by contact<strong>in</strong>g <strong>PHA</strong>.<br />
Vic Tapson, MD<br />
Editor-<strong>in</strong>-Chief<br />
years, research has been his passion—lead<strong>in</strong>g him to<br />
serve on the steer<strong>in</strong>g committee of the national registry<br />
and to play a key role <strong>in</strong> the growth of the 5,000-member<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Association (<strong>PHA</strong>), of which he<br />
is now president. “It was <strong>in</strong> the late 1970s that I discovered<br />
the NIH was start<strong>in</strong>g its patient registry <strong>in</strong> pulmonary<br />
hypertension so I applied to have our center enrolled<br />
as part of the registry,” he said. “Soon afterward, I was<br />
<strong>in</strong>vited to be on the steer<strong>in</strong>g committee for the patient<br />
registry at a time when we began collect<strong>in</strong>g data <strong>in</strong> an<br />
organized manner.” Jo<strong>in</strong><strong>in</strong>g the faculty at the <strong>University</strong><br />
of Ill<strong>in</strong>ois at Chicago, Dr Brundage teamed with Stuart<br />
Rich, MD, and Paul Levy, PhD, two lead<strong>in</strong>g <strong>in</strong>vestigators,<br />
as they explored the effects of high-dose calcium channel<br />
blockers <strong>in</strong> treat<strong>in</strong>g pulmonary hypertension. “This was<br />
the first breakthrough <strong>in</strong> the treatment because we found<br />
there was a percentage of patients who were helped.”<br />
In 1990 Dr Brundage was named chief of the<br />
Department of Cardiology at Harbor-UCLA Medical Center<br />
and became <strong>in</strong>volved <strong>in</strong> the early studies of <strong>in</strong>travenous<br />
prostacycl<strong>in</strong> therapy. Enroll<strong>in</strong>g 300 patients to receive<br />
what was a new <strong>in</strong>fusion therapy at the time, he was a<br />
coauthor of a major paper published <strong>in</strong> the Journal of the<br />
American College of Cardiology demonstrat<strong>in</strong>g the longterm<br />
survival benefits of prostacycl<strong>in</strong>. At that po<strong>in</strong>t<br />
(cont<strong>in</strong>ued on page 20)<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 3
The <strong>Pulmonary</strong><br />
<strong>Hypertension</strong><br />
Association<br />
Patients, family members,<br />
physicians and nurses provid<strong>in</strong>g<br />
hope, support, and education.<br />
Through research programs,<br />
<strong>PHA</strong> seeks better treatments<br />
and a cure.<br />
Jo<strong>in</strong> the <strong>PHA</strong> Today<br />
If we are go<strong>in</strong>g to beat this illness,<br />
we need you. Hundreds of physicians<br />
and hundreds more nurses are<br />
already part of our PH community.<br />
By jo<strong>in</strong><strong>in</strong>g today, you will be enrich<strong>in</strong>g<br />
your own access to <strong>in</strong>formation about<br />
an illness where treatment options<br />
are grow<strong>in</strong>g.<br />
Go to www.phassociation.org and<br />
click on the red “Jo<strong>in</strong> <strong>PHA</strong>” button.<br />
Visit us at www.phassociation.org<br />
<strong>PHA</strong>’s website is a gateway to the PH community. With the active support<br />
of <strong>PHA</strong>’s Scientific Leadership Council, it is also a grow<strong>in</strong>g source of medical<br />
<strong>in</strong>formation (www.phassociation.org/Medical) about pulmonary hypertension:<br />
• Complete copies of all issues of <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
• N<strong>in</strong>e consensus statements adopted by <strong>PHA</strong>’s Scientific<br />
Leadership Council:<br />
- Summary and explanation of common PH tests<br />
- Flolan guidel<strong>in</strong>es<br />
- Exercise recommendations<br />
- Sodium restriction recommendations<br />
- Recommendations on over-the-counter medications<br />
- Elective surgery<br />
- Travel recommendations<br />
- Referral recommendations<br />
• Answers to Frequently Asked Questions<br />
• PH physician list<strong>in</strong>gs<br />
• On-l<strong>in</strong>e survey of medical <strong>in</strong>terests<br />
• New: Cl<strong>in</strong>ical Trials section<br />
• New: <strong>Pulmonary</strong> <strong>Hypertension</strong> Resource Network (PHRN) <strong>in</strong>formation<br />
• New: Bibliography of PH articles<br />
Learn and teach with<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong>:<br />
A Patient’s Survival Guide<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong>: A Patient’s Survival Guide is now <strong>in</strong> its second edition.<br />
Many physicians and nurses order this publication <strong>in</strong> quantity for their own <strong>in</strong>formation<br />
and to give to their patients. The author, Gail Boyer Hayes, is a professional<br />
writer who has lived with PH for almost 20 years. Bruce Brundage, MD,<br />
past chairman of <strong>PHA</strong>’s Scientific Leadership Council and a physician with a<br />
deep knowledge of PH, edited the book for medical accuracy.<br />
Among the chapter head<strong>in</strong>gs <strong>in</strong> this 215-page book are:<br />
• What Is PH?<br />
• How Do I Know I’ve Really Got PH?<br />
• Who Gets PH?<br />
• PH Treatments<br />
• Children and PH<br />
• How to Take Your Medic<strong>in</strong>e<br />
• Liv<strong>in</strong>g with PH<br />
Also <strong>in</strong>cluded: Resource section, Bibliography, and Glossary<br />
Available for $25 per copy (or $15 for members), <strong>in</strong>clud<strong>in</strong>g shipp<strong>in</strong>g.<br />
Call 301-565-3004 or order on-l<strong>in</strong>e at www.phassociation.org<br />
(click on “Onl<strong>in</strong>e Store”)
Scleroderma-Associated <strong>Pulmonary</strong> <strong>Hypertension</strong>:<br />
Who’s at Risk and Why<br />
Karen A. Fagan, MD<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Center<br />
<strong>University</strong> of Colorado<br />
Health Sciences Center<br />
Denver, Colorado<br />
David H. Collier, MD<br />
Division of Rheumatology<br />
Denver Health Medical Center<br />
Denver, Colorado<br />
Introduction<br />
<strong>Pulmonary</strong> arterial hypertension is a life-threaten<strong>in</strong>g complication<br />
of several connective tissue diseases, <strong>in</strong>clud<strong>in</strong>g both diffuse<br />
and limited scleroderma (with a subgroup of limited scleroderma<br />
called the CREST syndrome), systemic lupus erythematosus<br />
(SLE), mixed connective tissue disease (MCTD), and<br />
less commonly, rheumatoid arthritis, and dermatomyositis/<br />
polymyositis (Table 1). This review will discuss the <strong>in</strong>cidence,<br />
potential etiologies, cl<strong>in</strong>ical presentation, and treatment options<br />
for patients with pulmonary hypertension and the scleroderma<br />
spectrum of diseases.<br />
Epidemiology<br />
<strong>Pulmonary</strong> hypertension complicates several of the connective<br />
tissue diseases (Table 1). Scleroderma is a progressive, multisystem<br />
disease manifested by connective tissue and vascular<br />
lesions <strong>in</strong> many organs, <strong>in</strong>clud<strong>in</strong>g lung, kidney, and sk<strong>in</strong>.<br />
<strong>Pulmonary</strong> manifestations <strong>in</strong>clude <strong>in</strong>terstitial fibrosis, pulmonary<br />
arterial hypertension, constriction of the chest wall<br />
due to sk<strong>in</strong> thicken<strong>in</strong>g, diaphragmatic dysfunction, and chronic<br />
aspiration due to esophageal dysmotility. 1 <strong>Pulmonary</strong> complications<br />
are the most frequent cause of death <strong>in</strong> patients<br />
with scleroderma, 1 and pulmonary vascular disease has a<br />
particularly adverse effect on prognosis. 2<br />
The <strong>in</strong>cidence of pulmonary hypertension varies between<br />
6% and 60% of patients with scleroderma. Up to 33% of<br />
patients with diffuse scleroderma have pulmonary hypertension,<br />
both isolated and <strong>in</strong> association with <strong>in</strong>terstitial lung disease.<br />
3-6 In patients with limited scleroderma, formerly referred<br />
to as CREST (calc<strong>in</strong>osis cutis, Raynaud’s phenomenon,<br />
esophageal dysmotility, sclerodactyly, and telangiectasias), up<br />
to 60% of patients have pulmonary hypertension. 4, 6-8 While<br />
not all patients have cl<strong>in</strong>ically significant pulmonary hypertension,<br />
two thirds of patients with scleroderma will have pathologic<br />
evidence of pulmonary vascular disease. 7, 9 Stupi et al<br />
reported two-year survival <strong>in</strong> patients with CREST without pulmonary<br />
hypertension to be greater than 80% while patients<br />
with pulmonary hypertension had a two-year survival of 40%. 8<br />
Sacks et al reported two-year survival of patients with pulmonary<br />
hypertension and either diffuse or limited scleroderma<br />
to be approximately 50%. 5 Koh et al reported 40% survival <strong>in</strong><br />
David B. Badesch, MD<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Center<br />
<strong>University</strong> of Colorado<br />
Health Sciences Center<br />
Denver, Colorado<br />
Table 1—Connective Tissue Diseases Associated<br />
with <strong>Pulmonary</strong> Arterial <strong>Hypertension</strong><br />
Scleroderma<br />
Diffuse<br />
Limited<br />
CREST<br />
Systemic lupus erythematosis<br />
Mixed connective tissue disease<br />
Rheumatoid arthritis<br />
Dermatomyositis/Polymyositis<br />
patients with scleroderma and pulmonary hypertension compared<br />
with higher survival <strong>in</strong> scleroderma patients without<br />
organ failure or with other lung <strong>in</strong>volvement (i.e. <strong>in</strong>terstitial<br />
lung disease) at two years. 2<br />
<strong>Pulmonary</strong> hypertension has been reported <strong>in</strong> 4% to 14%<br />
of patients with systemic SLE with an overall mortality rate of<br />
25% to 50% at two years from diagnosis of pulmonary hypertension.<br />
10-13 Patients with MCTD have features of several<br />
connective tissue diseases, <strong>in</strong>clud<strong>in</strong>g SLE, scleroderma,<br />
rheumatoid arthritis, and polymyositis. Most MCTD patients<br />
have either predom<strong>in</strong>antly SLE or scleroderma with a myositis<br />
overlap. The behavior of the disease therefore follows either<br />
a predom<strong>in</strong>antly SLE or a scleroderma pattern. The <strong>in</strong>cidence<br />
of pulmonary hypertension <strong>in</strong> patients with MCTD is not certa<strong>in</strong><br />
but one report found two thirds of patients with MCTD<br />
had evidence of pulmonary hypertension 14 and pulmonary<br />
hypertension has been frequently cited as a cause of death<br />
<strong>in</strong> patients with MCTD. 15 The high <strong>in</strong>cidence of pulmonary<br />
hypertension <strong>in</strong> MCTD is probably a result of the predom<strong>in</strong>ant<br />
scleroderma pattern of this disease <strong>in</strong> many patients with<br />
MCTD.<br />
Rheumatoid arthritis affects 5% of the population over<br />
age 65 and pulmonary complications <strong>in</strong>clude <strong>in</strong>terstitial pulmonary<br />
fibrosis, rheumatoid nodules, and pleural effusions.<br />
The <strong>in</strong>cidence of isolated pulmonary hypertension is not<br />
known. In a recent report, 21% of patients with rheumatoid<br />
arthritis without evidence of other pulmonary or cardiac dis-<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 5
ease had mild pulmonary hypertension. 16 The prognosis is<br />
not known. Other connective tissue diseases <strong>in</strong>clud<strong>in</strong>g dermatomyositis/polymyositis<br />
have been associated with pulmonary<br />
arterial hypertension but the <strong>in</strong>cidence and prognosis are<br />
not known. 17<br />
Pathogenesis<br />
The etiology of pulmonary hypertension <strong>in</strong> the scleroderma<br />
spectrum of diseases rema<strong>in</strong>s obscure. There appears to be<br />
direct <strong>in</strong>volvement of the pulmonary circulation with <strong>in</strong>timal<br />
proliferation and medial hypertrophy, similar to that seen <strong>in</strong><br />
primary pulmonary hypertension. 6-9, 18 Some cases may also<br />
be related to severe pulmonary parenchymal disease, such<br />
as <strong>in</strong>terstitial disease with hypoxemia. Additionally, diastolic<br />
dysfunction of the right and left ventricles has been seen <strong>in</strong><br />
patients with scleroderma and may contribute to pulmonary<br />
hypertension. 19<br />
Autoimmune processes have been implicated <strong>in</strong> the pathogenesis<br />
of pulmonary hypertension although the mechanism<br />
is not known. Positive ant<strong>in</strong>uclear antibodies are frequently<br />
found <strong>in</strong> pulmonary hypertension patients without a diagnosis<br />
of connective tissue disease and pulmonary hypertension can<br />
occur before the onset of an identifiable connective tissue disease.<br />
In patients with scleroderma, anticentromere and antihistone<br />
antibodies have been associated with vascular disease.<br />
Anticentromere antibodies are primarily seen <strong>in</strong> the limited<br />
form of systemic sclerosis. S<strong>in</strong>ce patients with the limited<br />
form of systemic sclerosis have a higher <strong>in</strong>cidence of pulmonary<br />
hypertension than do patients with diffuse disease,<br />
it is not surpris<strong>in</strong>g that anticentromere antibodies would be<br />
associated with a higher <strong>in</strong>cidence of pulmonary hypertension.<br />
Antifibrillar<strong>in</strong> antibodies (anti-U3-RNP) are frequently found<br />
<strong>in</strong> patients with scleroderma and are more common with diffuse<br />
scleroderma-associated pulmonary hypertension. 20 Antiendothelial<br />
antibodies (aECA) are present <strong>in</strong> 40% and 13%<br />
of patients with diffuse scleroderma and CREST, respectively,<br />
and are associated with a higher <strong>in</strong>cidence of pulmonary<br />
hypertension and digital <strong>in</strong>farcts. 21 Antifibrillar<strong>in</strong> antibodies<br />
and aECAs are also associated with pulmonary hypertension <strong>in</strong><br />
SLE. 22 In patients with scleroderma and pulmonary hypertension,<br />
especially when accompanied by HLA-B35 antigen, antitopoisomerase<br />
II-alpha antibodies are more common, as are<br />
antibodies to fibr<strong>in</strong>-bound tissue type plasm<strong>in</strong>ogen activator. 23<br />
Raynaud’s phenomenon, vasospasm of the arterioles <strong>in</strong> the<br />
distal systemic circulation, is commonly reported <strong>in</strong> patients<br />
with scleroderma. In one report, all patients with pulmonary<br />
hypertension and CREST had Raynaud’s, while 68% without<br />
pulmonary hypertension had Raynaud’s. 8 Raynaud’s is also<br />
common <strong>in</strong> patients with SLE and MCTD and pulmonary<br />
hypertension 11, 24 but only 10% to 14% of patients with primary<br />
pulmonary hypertension have Raynaud’s. 25 This observation<br />
has led to the “pulmonary Raynaud’s” hypothesis that<br />
vasospasm contributes to the development of pulmonary<br />
hypertension. 26<br />
Acute hypoxic pulmonary vasoconstriction may be more<br />
pronounced <strong>in</strong> patients with pulmonary hypertension and<br />
scleroderma than <strong>in</strong> patients with primary pulmonary hypertension.<br />
27 However, another report found that pulmonary<br />
vasospasm was not present <strong>in</strong> patients with Raynaud’s and<br />
6 <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
scleroderma without pulmonary hypertension. 28 In support<br />
of this hypothesis, patients with scleroderma have defective<br />
endothelial-dependent vasodilation15 and this may be related<br />
to decreased endothelial nitric oxide synthase (eNOS). 29<br />
Although controversial, decreased lung eNOS has been reported<br />
<strong>in</strong> severe primary pulmonary hypertension. 30 While the<br />
level of eNOS <strong>in</strong> connective tissue disease is not known,<br />
decreased production of lung nitric oxide has been found <strong>in</strong><br />
patients with scleroderma and pulmonary hypertension. 31<br />
Similarly, expression of prostacycl<strong>in</strong> synthase <strong>in</strong> pulmonary<br />
endothelium may be decreased <strong>in</strong> patients with severe connective<br />
tissue disease-associated pulmonary hypertension. 32<br />
Endothel<strong>in</strong>-1 is <strong>in</strong>creased <strong>in</strong> serum of patients with both<br />
diffuse and limited scleroderma33 and while endothel<strong>in</strong> levels<br />
correlate with survival <strong>in</strong> patients with scleroderma, 34 they are<br />
not higher <strong>in</strong> those with pulmonary hypertension. 33 In contrast,<br />
higher serum endothel<strong>in</strong> levels are found <strong>in</strong> patients<br />
with SLE-associated pulmonary hypertension than <strong>in</strong> nonpulmonary<br />
hypertensive SLE patients. 12 The role of endothel<strong>in</strong>-1<br />
<strong>in</strong> pulmonary hypertension has led to the use of endothel<strong>in</strong><br />
antagonists <strong>in</strong> treatment of patients with connective tissue<br />
disease-associated pulmonary hypertension. 35 Seroton<strong>in</strong> may<br />
also play a role <strong>in</strong> the pathogenesis of pulmonary hypertension.<br />
In patients with systemic sclerosis and Raynaud’s,<br />
platelet seroton<strong>in</strong> concentrations are decreased and serum<br />
36, 37<br />
levels are <strong>in</strong>creased.<br />
Cl<strong>in</strong>ical Presentation and Evaluation<br />
Dyspnea is the most common present<strong>in</strong>g symptom of scleroderma-associated<br />
pulmonary hypertension. The cl<strong>in</strong>ical evaluation<br />
is similar to that of patients with primary pulmonary<br />
hypertension. History and physical exam<strong>in</strong>ation often reveal<br />
f<strong>in</strong>d<strong>in</strong>gs of the underly<strong>in</strong>g connective tissue disease (ie,<br />
Raynaud’s, telangiectasias, rash, synovitis, <strong>in</strong>terstitial lung<br />
disease, etc). Decreased diffus<strong>in</strong>g capacity of the lung is the<br />
most common pulmonary function abnormality and should<br />
prompt an evaluation for both pulmonary vascular and <strong>in</strong>terstitial<br />
lung disease. 38 A diffus<strong>in</strong>g capacity of less than 40% of<br />
predicted for lung volume places the patient <strong>in</strong> a poor prognostic<br />
category. Echocardiography may be helpful <strong>in</strong> the evaluation<br />
of patients suspected of hav<strong>in</strong>g pulmonary hypertension<br />
as suggested by unexpla<strong>in</strong>ed dyspnea or an isolated<br />
reduction <strong>in</strong> diffus<strong>in</strong>g capacity.<br />
As previously discussed, patients with scleroderma should<br />
be considered an “at risk” group for the development of pulmonary<br />
hypertension, and echocardiography may reveal right<br />
ventricular hypertrophy and dilatation even before the onset of<br />
symptoms. 39 Ultimately, as with primary pulmonary hypertension,<br />
right-heart catheterization is needed to confirm the diagnosis,<br />
assess hemodynamic severity, and exclude other possible<br />
contribut<strong>in</strong>g factors, such as an occult congenital heart<br />
defect. While it is generally thought that patients with scleroderma-associated<br />
pulmonary hypertension are less likely to<br />
demonstrate a favorable response to vasodilator therapy than<br />
patients with primary pulmonary hypertension (<strong>in</strong> whom the<br />
response rate is approximately 20% to 25%), a hemodynamically<br />
monitored assessment of vasoreactivity is still advocated<br />
by some experts.
Table 2—Potential Therapeutic Options<br />
Vasodilators<br />
Calcium channel blockers<br />
Angiotens<strong>in</strong> convert<strong>in</strong>g enzyme <strong>in</strong>hibitors<br />
Alpha-adrenergic blockers<br />
Prostagland<strong>in</strong> preparations<br />
Intravenous epoprostenol<br />
Subcutaneous treprost<strong>in</strong>il<br />
Inhaled iloprost<br />
Inhaled nitric oxide<br />
Phosphodiesterase <strong>in</strong>hibitors<br />
Endothel<strong>in</strong> receptor antagonists<br />
Seroton<strong>in</strong> antagonists<br />
Immunosuppressive therapy<br />
Corticosteroids<br />
Cyclophosphamide<br />
Bone marrow transplantation<br />
Lung/Heart-lung transplantation<br />
Therapy<br />
Several therapeutic options are available for the treatment<br />
of scleroderma-associated pulmonary hypertension (Table 2).<br />
Oral vasodilators (calcium channel antagonists, angiotens<strong>in</strong><br />
convert<strong>in</strong>g enzyme <strong>in</strong>hibitors, and alpha-adrenergic antagonists)<br />
have been used to treat pulmonary hypertension <strong>in</strong> patients<br />
with scleroderma. Although it has been reported that<br />
calcium channel blockers have improved survival <strong>in</strong> some<br />
patients with scleroderma-associated pulmonary hypertension,<br />
40-42 it is generally acknowledged that only a small percentage<br />
of such patients respond favorably to these agents.<br />
Angiotens<strong>in</strong> convert<strong>in</strong>g enzyme <strong>in</strong>hibitors and an alpha-adrenergic<br />
blocker (prazos<strong>in</strong>) have also been used both acutely<br />
and over the long term <strong>in</strong> the treatment of connective tissue<br />
disease-associated pulmonary hypertension. 41,43<br />
In a randomized, multicenter study of cont<strong>in</strong>uously<br />
<strong>in</strong>travenously <strong>in</strong>fused epoprostenol we reported short-term<br />
improvement <strong>in</strong> patients with pulmonary hypertension due to<br />
scleroderma; 44 111 patients with pulmonary hypertension and<br />
the scleroderma spectrum of disease (70% limited disease,<br />
13% diffuse disease, 11% to 14% overlap syndrome, and 5%<br />
with features of scleroderma) were randomized to receive cont<strong>in</strong>uous<br />
<strong>in</strong>fusion of epoprostenol vs. conventional treatment for<br />
12 weeks. Epoprostenol improved exercise capacity, cardiopulmonary<br />
hemodynamics, New York Heart Association functional<br />
class, Borg dyspnea <strong>in</strong>dex, and likely Raynaud’s. However,<br />
there was no mortality benefit as had been seen <strong>in</strong> the same<br />
treatment duration with primary pulmonary hypertension, 45<br />
possibly because of the multisystem nature of this disease. 44<br />
It is important to po<strong>in</strong>t out that the study was not powered to<br />
detect a survival difference. Others have also found both short<br />
and long-term improvement with epoprostenol. 46,47 Long-term<br />
follow up of the patients <strong>in</strong> our study has suggested that<br />
epoprostenol may improve survival compared with historical<br />
controls. However, <strong>in</strong> general it appears as though survival/<br />
prognosis is worse for patients with scleroderma-associated<br />
pulmonary hypertension as compared with patients with<br />
primary pulmonary hypertension and needs further <strong>in</strong>vestigation.<br />
Treatment with epoprostenol <strong>in</strong> some patients has been<br />
associated with reports of pulmonary edema possibly result<strong>in</strong>g<br />
from pulmonary veno-occlusive disease or pulmonary capillary<br />
hemangiomatosis. 48 Although very rare, pulmonary veno-occlusive<br />
disease may be more common <strong>in</strong> patients with connective<br />
tissue disease. 49<br />
Increas<strong>in</strong>g evidence has suggested the importance of<br />
endothel<strong>in</strong>-1 <strong>in</strong> the pathogenesis of pulmonary hypertension.<br />
In a multicenter, randomized, double-bl<strong>in</strong>ded placebo controlled<br />
trial of the endothel<strong>in</strong> receptor antagonist bosentan<br />
(Tracleer ® ) for the treatment of pulmonary arterial hypertension,<br />
213 patients with pulmonary hypertension, either primary<br />
or due to connective tissue disease (scleroderma and<br />
lupus), were randomized to receive placebo or bosentan at<br />
125 or 250 mg orally twice daily. 35 After 16 weeks, distance<br />
walked <strong>in</strong> six m<strong>in</strong>utes, functional class, Borg dyspnea <strong>in</strong>dex,<br />
and time to cl<strong>in</strong>ical worsen<strong>in</strong>g improved <strong>in</strong> patients receiv<strong>in</strong>g<br />
either dose of bosentan. In contrast to the improvement <strong>in</strong><br />
patients with primary pulmonary hypertension, bosentan prevented<br />
the deterioration <strong>in</strong> six-m<strong>in</strong>ute walk compared with<br />
placebo. This suggested that patients with scleroderma did<br />
less well overall. Nevertheless, relative stability may represent<br />
a favorable outcome <strong>in</strong> a disease with an otherwise very<br />
poor prognosis. Bosentan has been associated with a dosedependent<br />
<strong>in</strong>crease <strong>in</strong> liver function tests, and monthly<br />
follow-up of these tests is required by the Food and Drug<br />
Adm<strong>in</strong>istration. Other potential side effects are thought to<br />
<strong>in</strong>clude mild anemia, fluid retention, teratogenicity, and possibly<br />
testicular dysfunction and male <strong>in</strong>fertility. Even <strong>in</strong> light of<br />
these potential adverse effects, the development of this oral<br />
therapy is thought to represent a significant advance.<br />
Various prostacycl<strong>in</strong> analogues and delivery systems have<br />
been recently studied. Inhaled iloprost, a stable analogue of<br />
epoprostenol, was studied <strong>in</strong> a large placebo-controlled trial<br />
compar<strong>in</strong>g <strong>in</strong>haled iloprost with placebo <strong>in</strong> patients with<br />
severe pulmonary hypertension. Iloprost improved six-m<strong>in</strong>ute<br />
walk test results, functional status, and hemodynamics after<br />
12 weeks of treatment. 50 The effect was greatest <strong>in</strong> patients<br />
with primary pulmonary hypertension. Comb<strong>in</strong>ation with a<br />
phosphodiesterase <strong>in</strong>hibitor appears to <strong>in</strong>crease the effectiveness<br />
of <strong>in</strong>haled iloprost <strong>in</strong> patients with pulmonary hypertension.<br />
51 Treprost<strong>in</strong>il, a stable prostacycl<strong>in</strong> analogue adm<strong>in</strong>istered<br />
subcutaneously, was recently approved for use <strong>in</strong><br />
patients with pulmonary arterial hypertension with efficacy at<br />
the highest doses of the drug. 52 Beraprost sodium, an orally<br />
bioactive prostacycl<strong>in</strong> analogue, improved six-m<strong>in</strong>ute walk<br />
distance <strong>in</strong> patients with primary pulmonary hypertension<br />
compared with patients with connective tissue disease. 53<br />
Although nitric oxide has utility <strong>in</strong> acute pulmonary<br />
vasodilator test<strong>in</strong>g <strong>in</strong> patients with scleroderma, there have<br />
not been any reports of its long-term use <strong>in</strong> the treatment of<br />
scleroderma-associated pulmonary hypertension. The selective<br />
seroton<strong>in</strong> receptor 2 antagonist ketanser<strong>in</strong> acutely improved<br />
pulmonary artery pressure and cardiac output <strong>in</strong> patients with<br />
scleroderma-associated pulmonary hypertension 54 while sar-<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 7
pogrelate, another receptor 2 antagonist, adm<strong>in</strong>istered orally<br />
for 12 months, decreased mean pulmonary arterial pressure<br />
and <strong>in</strong>creased right ventricular ejection fraction. 55 These<br />
reports suggest a role for seroton<strong>in</strong> <strong>in</strong> the pathogenesis of<br />
scleroderma-associated pulmonary arterial hypertension,<br />
although a randomized, controlled trial has not been done.<br />
Corticosteroids with and without cyclophosphamide, 13<br />
long-term plasma exchange, 56 and autologous stem cell transplantation<br />
57 have been reported to improve or stabilize pulmonary<br />
hypertension <strong>in</strong> patients with scleroderma. However,<br />
these represent case reports or retrospective case studies and<br />
no prospective study of immunosuppressive therapy has been<br />
completed <strong>in</strong> patients with connective tissue disease-related<br />
pulmonary hypertension. Use of immunosuppressive therapy<br />
may be more successful <strong>in</strong> patients with SLE than <strong>in</strong> those<br />
with scleroderma.<br />
Surgical treatment, <strong>in</strong>clud<strong>in</strong>g atrial septostomy 58 and lung<br />
or heart-lung transplantation may be considered for patients<br />
with severe pulmonary arterial hypertension <strong>in</strong> association<br />
with connective tissue disease. Survival <strong>in</strong> patients with connective<br />
tissue disease-associated pulmonary hypertension who<br />
undergo lung or heart-lung transplantation is not different<br />
from that <strong>in</strong> patients with primary pulmonary hypertension. 59<br />
Lung transplantation may also be of benefit <strong>in</strong> patients with<br />
severe fibrotic lung disease. Appropriate patient selection is<br />
important, though, and lung transplantation may be relatively<br />
contra<strong>in</strong>dicated <strong>in</strong> patients with significant esophageal dysmotility<br />
or renal dysfunction.<br />
Summary<br />
Patients with scleroderma are at <strong>in</strong>creased risk for the development<br />
of pulmonary hypertension, and the development of<br />
unexpla<strong>in</strong>ed dyspnea or an isolated decrease <strong>in</strong> diffus<strong>in</strong>g<br />
capacity should prompt evaluation. Echocardiography is often<br />
helpful <strong>in</strong> this situation. Because the prognosis of untreated<br />
pulmonary hypertension occurr<strong>in</strong>g <strong>in</strong> the sett<strong>in</strong>g of scleroderma<br />
is generally quite poor, vigilance is required on the part of<br />
physicians follow<strong>in</strong>g this “at risk” group of patients. The past<br />
decade has seen important advances <strong>in</strong> the treatment of<br />
pulmonary arterial hypertension, <strong>in</strong>clud<strong>in</strong>g <strong>in</strong>travenous epoprostenol,<br />
oral bosentan, and subcutaneously <strong>in</strong>fused treprost<strong>in</strong>il.<br />
As new therapies are developed for the treatment of pulmonary<br />
arterial hypertension, it is essential that patients with<br />
scleroderma-related disease are <strong>in</strong>cluded <strong>in</strong> cl<strong>in</strong>ical trials. PH<br />
Acknowledgement<br />
Portions of this report are similar to upcom<strong>in</strong>g articles written by the<br />
same authors <strong>in</strong> the 2 nd Edition of <strong>Pulmonary</strong> Circulation edited by<br />
Drs Andrew Peacock and Lewis J. Rub<strong>in</strong> and <strong>in</strong> Progress <strong>in</strong><br />
Cardiovascular Diseases.<br />
References<br />
1. M<strong>in</strong>ai OA, Dweik RA, Arroliga AC. Manifestations of scleroderma pulmonary<br />
disease. Cl<strong>in</strong> Chest Med 1998;19(4):713-31, viii-ix.<br />
2. Koh ET, Lee P, Gladman DD, et al. <strong>Pulmonary</strong> hypertension <strong>in</strong> systemic<br />
sclerosis: an analysis of 17 patients. Br J Rheumatol 1996;35<br />
(10):989-93.<br />
8 <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
3. Battle RW, Davitt MA, Cooper SM, et al. Prevalence of pulmonary<br />
hypertension <strong>in</strong> limited and diffuse scleroderma. Chest 1996;110<br />
(6):1515-9.<br />
4. MacGregor AJ, Canavan R, Knight C, et al. <strong>Pulmonary</strong> hypertension <strong>in</strong><br />
systemic sclerosis: risk factors for progression and consequences for survival.<br />
Rheumatology (Oxford) 2001;40(4):453-9.<br />
5. Sacks DG, Okano Y, Steen VD, et al. Isolated pulmonary hypertension<br />
<strong>in</strong> systemic sclerosis with diffuse cutaneous <strong>in</strong>volvement: association<br />
with serum anti-U3RNP antibody. J Rheumatol 1996;23(4):639-42.<br />
6. Ungerer RG, Tashk<strong>in</strong> DP, Furst D, et al. Prevalence and cl<strong>in</strong>ical correlates<br />
of pulmonary arterial hypertension <strong>in</strong> progressive systemic sclerosis.<br />
Am J Med 1983;75(1):65-74.<br />
7. Salerni RG, Rodnan P, Leon DF, et al. <strong>Pulmonary</strong> hypertension <strong>in</strong> the<br />
CREST syndrome variant of progressive systemic sclerosis (scleroderma).<br />
Ann Intern Med 1977;86(4):394-9.<br />
8. Stupi AM, Steen VD, Owens GR, et al. <strong>Pulmonary</strong> hypertension <strong>in</strong> the<br />
CREST syndrome variant of systemic sclerosis. Arthritis Rheum 1986;29<br />
(4):515-24.<br />
9. Young RH, Mark GJ. <strong>Pulmonary</strong> vascular changes <strong>in</strong> scleroderma. Am<br />
J Med 1978;64(6):998-1004.<br />
10. Asherson RA, Higenbottam TW, D<strong>in</strong>h Xuan AT, et al. <strong>Pulmonary</strong><br />
hypertension <strong>in</strong> a lupus cl<strong>in</strong>ic: experience with twenty-four patients.<br />
J Rheumatol 1990;17(10):1292-8.<br />
11. Li EK, Tam LS. <strong>Pulmonary</strong> hypertension <strong>in</strong> systemic lupus erythematosus:<br />
cl<strong>in</strong>ical association and survival <strong>in</strong> 18 patients. J Rheumatol<br />
1999;26(9):1923-9.<br />
12. Shen JY, Chen SL, Wu YX, et al. <strong>Pulmonary</strong> hypertension <strong>in</strong> systemic<br />
lupus erythematosus. Rheumatol Int 1999;18(4):147-51.<br />
13. Tanaka E, Harigai M, Tanaka M, et al. <strong>Pulmonary</strong> hypertension <strong>in</strong><br />
systemic lupus erythematosus: evaluation of cl<strong>in</strong>ical characteristics and<br />
response to immunosuppressive treatment. J Rheumatol 2002;29(2):<br />
282-7.<br />
14. Prakash UB. Respiratory complications <strong>in</strong> mixed connective tissue<br />
disease. Cl<strong>in</strong> Chest Med 1998;19(4):733-46, ix.<br />
15. Burdt MA, Hoffman RW, Deutscher SL, et al. Long-term outcome <strong>in</strong><br />
mixed connective tissue disease: longitud<strong>in</strong>al cl<strong>in</strong>ical and serologic f<strong>in</strong>d<strong>in</strong>gs.<br />
Arthritis Rheum 1999;42(5):899-909.<br />
16. Dawson JK, Goodson NG, Graham DR, et al. Raised pulmonary artery<br />
pressures measured with Doppler echocardiography <strong>in</strong> rheumatoid arthritis<br />
patients. Rheumatology (Oxford) 2000;39(12):1320-5.<br />
17. Denbow CE, Lie JT, Tancredi RG, et al. Cardiac <strong>in</strong>volvement <strong>in</strong><br />
polymyositis: a cl<strong>in</strong>icopathologic study of 20 autopsied patients. Arthritis<br />
Rheum 1979;22(10):1088-92.<br />
18. Yousem SA. The pulmonary pathologic manifestations of the CREST<br />
syndrome. Hum Pathol 1990;21(5):467-74.<br />
19. Giunta A, Tirri E, Maione S, et al. Right ventricular diastolic abnormalities<br />
<strong>in</strong> systemic sclerosis. Relation to left ventricular <strong>in</strong>volvement and<br />
pulmonary hypertension. Ann Rheum Dis 2000;59(2):94-8.<br />
20. Tormey VJ, Bunn CC, Denton CP, et al. Anti-fibrillar<strong>in</strong> antibodies <strong>in</strong><br />
systemic sclerosis. Rheumatology (Oxford) 2001;40(10):1157-62.<br />
21. Negi VS, Tripathy NK, Misra R, et al. Antiendothelial cell antibodies<br />
<strong>in</strong> scleroderma correlate with severe digital ischemia and pulmonary arterial<br />
hypertension. J Rheumatol 1998;25(3):462-6.<br />
22. Yoshio T, Masuyama J, Sumiya M, et al. Antiendothelial cell antibodies<br />
and their relation to pulmonary hypertension <strong>in</strong> systemic lupus erythematosus.<br />
J Rheumatol 1994;21(11):2058-63.<br />
23. Grigolo B, Mazzetti I, Meliconi R, et al. Anti-topoisomerase II alpha<br />
autoantibodies <strong>in</strong> systemic sclerosis- association with pulmonary hypertension<br />
and HLA-B35. Cl<strong>in</strong> Exp Immunol 2000;121(3):539-43.<br />
24. Ueda N, Mimura K, Maeda H, et al. Mixed connective tissue disease<br />
with fatal pulmonary hypertension and a review of literature. Virchows<br />
Arch A Pathol Anat Histopathol 1984;404(4):335-40.<br />
25. Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension.<br />
A national prospective study. Ann Intern Med 1987;107(2):<br />
216-23.<br />
26. Fahey PJ, Utell MJ, Condemi JJ, et al. Raynaud's phenomenon of the<br />
lung. Am J Med 1984;76(2):263-9.
27. Morgan JM, Griffiths M, du Bois RM, et al. Hypoxic pulmonary vasoconstriction<br />
<strong>in</strong> systemic sclerosis and primary pulmonary hypertension.<br />
Chest 1991;99(3):551-6.<br />
28. Shuck JW, Oetgen WJ, Tesar JT. <strong>Pulmonary</strong> vascular response dur<strong>in</strong>g<br />
Raynaud's phenomenon <strong>in</strong> progressive systemic sclerosis. Am J Med<br />
1985;78(2):221-7.<br />
29. Romero LI, Zhang DN, Cooke JP, et al. Differential expression of<br />
nitric oxide by dermal microvascular endothelial cells from patients with<br />
scleroderma. Vasc Med 2000;5(3):147-58.<br />
30. Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase<br />
<strong>in</strong> the lungs of patients with pulmonary hypertension. N Engl J<br />
Med 1995;333(4):214-21.<br />
31. Rolla G, Colagrande P, Scappaticci E, et al. Exhaled nitric oxide <strong>in</strong><br />
systemic sclerosis: relationships with lung <strong>in</strong>volvement and pulmonary<br />
hypertension. J Rheumatol 2000;27(7):1693-8.<br />
32. Tuder RM, Cool CD, Geraci MW, et al. Prostacycl<strong>in</strong> synthase expression<br />
is decreased <strong>in</strong> lungs from patients with severe pulmonary hypertension.<br />
Am J Respir Crit Care Med 1999;159(6):1925-32.<br />
33. Morelli S, Ferri C, Polett<strong>in</strong>i E, et al. Plasma endothel<strong>in</strong>-1 levels, pulmonary<br />
hypertension, and lung fibrosis <strong>in</strong> patients with systemic sclerosis.<br />
Am J Med 1995;99(3):255-60.<br />
34. Galie N, Bacchi-Reggiani GF, et al. Relation of endothel<strong>in</strong>-1 to survival<br />
<strong>in</strong> patients with primary pulmonary hypertension. European Journal<br />
of Cl<strong>in</strong>ical Investigation 1996;26(suppl 1):48.<br />
35. Rub<strong>in</strong> LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary<br />
arterial hypertension. N Engl J Med 2002;346(12):896-903.<br />
36. Klimiuk PS, Grennan A, We<strong>in</strong>kove C, et al. Platelet seroton<strong>in</strong> <strong>in</strong> systemic<br />
sclerosis. Ann Rheum Dis 1989;48(7):586-9.<br />
37. Biondi ML, Maras<strong>in</strong>i B, Bianchi E, et al. Plasma free and<br />
<strong>in</strong>traplatelet seroton<strong>in</strong> <strong>in</strong> patients with Raynaud's phenomenon. Int J<br />
Cardiol 1988;19(3):335-9.<br />
38. Scheja A, Akesson A, Wollmer P, et al. Early pulmonary disease <strong>in</strong><br />
systemic sclerosis: a comparison between carbon monoxide transfer factor<br />
and static lung compliance. Ann Rheum Dis 1993;52(10):725-9.<br />
39. Denton CP, Cailes JB, Phillips GD, et al. Comparison of Doppler<br />
echocardiography and right heart catheterization to assess pulmonary<br />
hypertension <strong>in</strong> systemic sclerosis. Br J Rheumatol 1997;36(2):239-43.<br />
40. Alpert MA, Pressly TA, Mukerji V, et al. Acute and long-term effects<br />
of nifedip<strong>in</strong>e on pulmonary and systemic hemodynamics <strong>in</strong> patients with<br />
pulmonary hypertension associated with diffuse systemic sclerosis, the<br />
CREST syndrome and mixed connective tissue disease. Am J Cardiol<br />
1991;68(17):1687-91.<br />
41. Glikson M, Pollack A, Dresner-Feig<strong>in</strong> R, et al. Nifedip<strong>in</strong>e and prazos<strong>in</strong><br />
<strong>in</strong> the management of pulmonary hypertension <strong>in</strong> CREST syndrome.<br />
Chest 1990;98(3):759-61.<br />
42. Sh<strong>in</strong>ohara S, Murata I, Yamada H, et al. Comb<strong>in</strong>ed effects of diltiazem<br />
and oxygen <strong>in</strong> pulmonary hypertension of mixed connective tissue<br />
disease. J Rheumatol 1994;21(9):1763-5.<br />
43. Alpert MA, Pressly TA, Mukerji V, et al. Short- and long-term hemodynamic<br />
effects of captopril <strong>in</strong> patients with pulmonary hypertension and<br />
selected connective tissue disease. Chest 1992;102(5):1407-12.<br />
44. Badesch DB, Tapson VF, McGoon MD, et al. Cont<strong>in</strong>uous <strong>in</strong>travenous<br />
epoprostenol for pulmonary hypertension due to the scleroderma spectrum<br />
of disease. A randomized, controlled trial. Ann Intern Med 2000;<br />
132(6):425-34.<br />
45. Barst RJ, Rub<strong>in</strong> LJ, Long WA, et al. A comparison of cont<strong>in</strong>uous<br />
<strong>in</strong>travenous epoprostenol (prostacycl<strong>in</strong>) with conventional therapy for<br />
primary pulmonary hypertension. The Primary <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
Study Group. 1996; N Engl J Med 334(5):296-302.<br />
46. Humbert M, Sanchez O, Fartoukh M, et al. Treatment of severe pulmonary<br />
hypertension secondary to connective tissue diseases with cont<strong>in</strong>uous<br />
IV epoprostenol (prostacycl<strong>in</strong>). Chest 1998;114(1 Suppl):80S-82S.<br />
47. Kl<strong>in</strong>gs ES, Hill NS, Ieong MH, et al. Systemic sclerosis-associated<br />
pulmonary hypertension: short- and long- term effects of epoprostenol<br />
(prostacycl<strong>in</strong>). Arthritis Rheum 1999;42(12):2638-45.<br />
48. Resten A, Maitre S, Humbert M, et al. <strong>Pulmonary</strong> arterial hypertension:<br />
th<strong>in</strong>-section CT predictors of epoprostenol therapy failure. Radiology<br />
2002;222(3):782-8.<br />
49. Mandel J, Mark EJ, Hales CA. <strong>Pulmonary</strong> veno-occlusive disease. Am<br />
J Respir Crit Care Med 2000;162(5):1964-73.<br />
50. Olschewski H, Simonneau G, Galie N, et al. for the Aerosolized<br />
Iloprost Randomized Study Group. Inhaled Iloprost for Severe <strong>Pulmonary</strong><br />
<strong>Hypertension</strong>. N Eng J Med 2002;347(5):322-329.<br />
51. Ghofrani HA, Wiedemann R, Rose F, et al. Comb<strong>in</strong>ation therapy with<br />
oral sildenafil and <strong>in</strong>haled iloprost for severe pulmonary hypertension.<br />
Ann Intern Med 2002;136(7):515-22.<br />
52. Simonneau G, Barst RJ, Galie N, et al. Cont<strong>in</strong>uous subcutaneous<br />
<strong>in</strong>fusion of treprost<strong>in</strong>il, a prostacycl<strong>in</strong> analogue, <strong>in</strong> patients with pulmonary<br />
arterial hypertension: a double-bl<strong>in</strong>d, randomized, placebo-controlled<br />
trial. Am J Respir Crit Care Med 2002;165(6):800-4.<br />
53. Galie N, Humbert M, Vachiery JL, et al. Effects of beraprost sodium,<br />
an oral prostacycl<strong>in</strong> analogue, <strong>in</strong> patients with pulmonary arterial hypertension:<br />
a randomized, double-bl<strong>in</strong>d, placebo-controlled trial. J Am Coll<br />
Cardiol 2002;39(9):1496-502.<br />
54. Seibold JR, Molony RR, Turkevich D, et al. Acute hemodynamic<br />
effects of ketanser<strong>in</strong> <strong>in</strong> pulmonary hypertension secondary to systemic<br />
sclerosis. J Rheumatol 1987;14(3):519-24.<br />
55. Kato S, Kishiro I, Machida M, et al. Suppressive effect of sarpogrelate<br />
hydrochloride on respiratory failure and right ventricular failure with<br />
pulmonary hypertension <strong>in</strong> patients with systemic sclerosis. J Int Med<br />
Res 2000;28(6):258-68.<br />
56. Ferri C, Emd<strong>in</strong> M, Stor<strong>in</strong>o FA, et al. Isolated pulmonary hypertension<br />
<strong>in</strong> diffuse cutaneous systemic sclerosis successfully treated with longterm<br />
plasma exchange. Scand J Rheumatol 2000;29(3):198-200.<br />
57. B<strong>in</strong>ks M, Passweg JR, Furst D, et al. Phase I/II trial of autologous<br />
stem cell transplantation <strong>in</strong> systemic sclerosis: procedure related mortality<br />
and impact on sk<strong>in</strong> disease. Ann Rheum Dis 2001;60(6):577-84.<br />
58. Allcock RJ, O'Sullivan JJ, Corris PA. Palliation of systemic sclerosisassociated<br />
pulmonary hypertension by atrial septostomy. Arthritis Rheum<br />
2001;44(7):1660-2.<br />
59. Rosas VJ, Conte V, Yang SC, et al. Lung transplantation and systemic<br />
sclerosis. Ann Transplant 2000;5(3):38-43.<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 9
Cardiac Catheterization <strong>in</strong> <strong>Pulmonary</strong> Arterial <strong>Hypertension</strong>:<br />
A Guide to Proper Use<br />
Ronald J. Oudiz, MD<br />
Assistant Professor of Medic<strong>in</strong>e<br />
UCLA School of Medic<strong>in</strong>e<br />
Director, Liu Center for <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
Harbor-UCLA Medical Center<br />
Torrance, California<br />
This article will discuss several features of cardiac catheterization,<br />
specifically right-heart catheterization, as they relate to<br />
patients with pulmonary arterial hypertension (PAH).<br />
The accepted gold standard def<strong>in</strong>ition of pulmonary hypertension<br />
is def<strong>in</strong>ed by most experts as a mean pulmonary arterial<br />
pressure of ≥ 25 mmHg, with a concomitant pulmonary<br />
capillary wedge (PCW) pressure of ≤15 mmHg, and pulmonary<br />
vascular resistance of >3 Wood units. These criteria are<br />
derived from the National Institutes of Health registry of patients<br />
with primary pulmonary hypertension. 1 Thus, by def<strong>in</strong>ition,<br />
cardiac catheterization is required to def<strong>in</strong>itively establish<br />
the diagnosis of PAH.<br />
Cardiac catheterization should be considered essential for<br />
document<strong>in</strong>g of hemodynamic severity, as well as complet<strong>in</strong>g<br />
a standard workup for pulmonary hypertension. The <strong>in</strong>formation<br />
obta<strong>in</strong>ed from cardiac catheterization <strong>in</strong> comb<strong>in</strong>ation with<br />
cl<strong>in</strong>ical f<strong>in</strong>d<strong>in</strong>gs can be used to monitor therapeutic and<br />
adverse effects of medical <strong>in</strong>terventions.<br />
Measurement of hemodynamics <strong>in</strong> patients with PAH via<br />
cardiac catheterization can also provide added prognostic<br />
value. For example, <strong>in</strong> patients with primary pulmonary hypertension<br />
whose mean right atrial pressure was
Precautions<br />
When plann<strong>in</strong>g cardiac catheterization for a patient with suspected<br />
PAH, it is important to understand the risks associated<br />
with the procedure, and to have an emergency treatment plan<br />
<strong>in</strong> place should these risks occur. In addition, the desired<br />
measurements should be planned <strong>in</strong> advance, with careful<br />
consideration of the specific operational procedures that are<br />
to be done dur<strong>in</strong>g the procedure.<br />
Cl<strong>in</strong>icians should be very familiar with how to <strong>in</strong>terpret the<br />
measurements obta<strong>in</strong>ed at cardiac catheterization, and be<br />
able to troubleshoot suspected <strong>in</strong>accuracies. Anticipation of<br />
complications and unexpected f<strong>in</strong>d<strong>in</strong>gs is essential, so that<br />
immediate action can be taken. F<strong>in</strong>ally, the cl<strong>in</strong>ician must<br />
cont<strong>in</strong>uously scrut<strong>in</strong>ize the f<strong>in</strong>d<strong>in</strong>gs and question the measurements<br />
for both accuracy and cl<strong>in</strong>ical relevance.<br />
Patients with PAH may present with relatively few physical<br />
signs of PAH, yet have significant cardiovascular abnormalities.<br />
These patients, with “compensated right-heart failure,”<br />
can easily decompensate when subjected to the stressors of<br />
cardiac catheterization. Despite these risks, however, cardiac<br />
catheterization is safe if appropriate precautions are carried<br />
out.<br />
• Staff experience – The physician and nurs<strong>in</strong>g and technical<br />
staff must all be familiar with the diagnosis and management<br />
of PAH and with the catheterization laboratory equipment.<br />
The staff must be meticulous about flush<strong>in</strong>g and level<strong>in</strong>g<br />
the pressure transducers and flush<strong>in</strong>g the catheter to<br />
ensure that accurate measurements are recorded.<br />
• Patient sedation – It is generally recommended that<br />
adult patients be kept awake dur<strong>in</strong>g catheterization. However,<br />
it is important that anxiety, which may <strong>in</strong>duce tachycardia and<br />
hemodynamic embarrassment, be controlled. Small doses of<br />
benzodiazep<strong>in</strong>es are useful for controll<strong>in</strong>g anxiety. Close attention<br />
to cont<strong>in</strong>uous pulse oximetry is required, however, as<br />
hypoxemia dur<strong>in</strong>g catheterization is not uncommon.<br />
• Atrial and ventricular ectopy – As the catheter is manipulated<br />
<strong>in</strong>to positions <strong>in</strong> the right atrium and ventricle, ectopic<br />
electrical activity is common. Usually, atrial premature beats<br />
and ventricular ectopic beats are brief and self-limited.<br />
Susta<strong>in</strong>ed activity <strong>in</strong>clud<strong>in</strong>g atrial and ventricular tachycardia<br />
may occur, however. Immediate reposition<strong>in</strong>g or removal of<br />
the catheter is required <strong>in</strong> these <strong>in</strong>stances, and antiarrhythmic<br />
therapy should always be available should the arrhythmia persist.<br />
• Bradyarrhythmias – One of the most troublesome complications<br />
of cardiac catheterization <strong>in</strong> patients with PAH is the<br />
development of vagally mediated bradycardia and hypotension.<br />
Often, an anxious or sensitive patient may develop<br />
<strong>in</strong>creased vagal tone 1) on view<strong>in</strong>g the catheterization <strong>in</strong>struments<br />
or dur<strong>in</strong>g local anesthetic <strong>in</strong>fusion; 2) on <strong>in</strong>sertion of<br />
the catheter; or 3) on removal of the catheter. When these<br />
“vagal episodes” occur, profound bradycardia and hypotension<br />
often ensue with<strong>in</strong> 30 to 60 seconds. It can be extremely difficult<br />
to resuscitate such a patient. Therefore, it is imperative<br />
that a vagal episode is anticipated <strong>in</strong> all patients, and that it<br />
is recognized and treated with atrop<strong>in</strong>e early <strong>in</strong> its course.<br />
This author always keeps an open vial of atrop<strong>in</strong>e at the bedside<br />
before, dur<strong>in</strong>g, and after cardiac catheterization of a<br />
patient with pulmonary hypertension.<br />
16 <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
<strong>Pulmonary</strong> Artery Pressure<br />
(mmHg)<br />
80<br />
70<br />
60<br />
50<br />
A B<br />
Patient #8 Patient #6<br />
• Reliability of measurements – Cardiac catheterization<br />
measurements should be made preferably when the patient<br />
is sup<strong>in</strong>e, with anxiety m<strong>in</strong>imized (see above), and at steady<br />
state. Spontaneous variation <strong>in</strong> hemodynamics over time is<br />
a known shortcom<strong>in</strong>g of cardiac catheterization (Figure 2), 5<br />
and thus great care should be taken to ensure that all measurements<br />
are taken <strong>in</strong> close proximity of each other. In general,<br />
wait<strong>in</strong>g at least 15 m<strong>in</strong>utes after catheter <strong>in</strong>sertion is<br />
advisable. Hemodynamic measurements should then be<br />
obta<strong>in</strong>ed as close together as possible.<br />
Choice of Venous Access Sites<br />
Commonly, the right <strong>in</strong>ternal jugular ve<strong>in</strong> is used for <strong>in</strong>sertion<br />
of a venous sheath through which the pulmonary artery<br />
catheter is passed. Other sites can be advantageous, depend<strong>in</strong>g<br />
on the situation (Table 1). For a patient’s <strong>in</strong>itial catheterization,<br />
use of the femoral ve<strong>in</strong>s for catheterization may be<br />
preferred, because it allows the greatest flexibility with which<br />
the cl<strong>in</strong>ician can perform the most thorough evaluation. This<br />
is especially important for exclud<strong>in</strong>g left heart pathology when<br />
direct measuremebnt of left ventricular end diastolic pressure<br />
is necessary.<br />
Measurements to Record<br />
Standard right-heart catheterization measurements (Figure 3)<br />
<strong>in</strong>clude:<br />
• right atrial • pulmonary arterial (PA)<br />
pressure (RAP) (“mixed venous”) saturation<br />
• right ventricular • superior vena cava<br />
pressure (RVP) (SVC) saturation*<br />
• pulmonary arterial • <strong>in</strong>ferior vena cava<br />
pressure (PAP) (IVC) saturation*<br />
• pulmonary capillary • right atrial (RA)<br />
wedge pressure (PCWP) saturation*<br />
• systemic arterial • right ventricular<br />
pressure (BP) and heart<br />
rate<br />
• cardiac output (CO)<br />
• pulmonary arterial<br />
vasoreactivity<br />
(RV) saturation*<br />
*When <strong>in</strong>dicated.<br />
1 2 3 4 5 6<br />
Hour<br />
19<br />
17<br />
15<br />
13<br />
Total <strong>Pulmonary</strong> Resisitance<br />
(units)<br />
<strong>Pulmonary</strong> Artery Pressure<br />
(mmHg)<br />
70<br />
60<br />
50<br />
40<br />
1 2 3 4 5 6<br />
Hour<br />
Fig. 2—Spontaneous variation <strong>in</strong> pulmonary arterial hemodynamics<br />
over time.<br />
5.0<br />
4.0<br />
3.0<br />
Cardiac Output (L / m<strong>in</strong>)
Table—Common Venous Access Sites<br />
Site Advantages Disadvantages Complications<br />
Right <strong>in</strong>ternal jugular ve<strong>in</strong> Facilitates pulmonary artery access; Cutaneous access Hematoma, pneumothorax,<br />
proximity to heart; may not need can be difficult tracheal obstruction<br />
fluoroscopy<br />
Left subclavian ve<strong>in</strong> Facilitates pulmonary artery access; Vascular control of bleed<strong>in</strong>g Pneumothorax, hemothorax<br />
proximity to heart difficult<br />
Femoral ve<strong>in</strong>s Easiest to cannulate; easiest for Most problematic for Hematoma<br />
vascular control of bleed<strong>in</strong>g pulmonary artery access; small<br />
risk of <strong>in</strong>fection; limits patient<br />
mobility; fluoroscopy required<br />
Fig. 3—Upper panel: Record<strong>in</strong>gs of <strong>in</strong>dividual hemodynamic measurements<br />
dur<strong>in</strong>g right-heart catheterization <strong>in</strong> a normal patient, and correspond<strong>in</strong>g<br />
anatomic locations. Lower panel: Expanded record<strong>in</strong>gs of<br />
right atrial pressure and pulmonary capillary wedge pressure (Wedge).<br />
In the Wedge record<strong>in</strong>g, the presence of a waves and v waves is<br />
supportive evidence of reliable balloon occlusion measurement,<br />
and accurate estimation of left atrial pressure. (Images courtesy<br />
of Blaufuss Multimedia Laboratories, San Francisco, CA.)<br />
Normal pressure waveforms are shown <strong>in</strong> Figure 3. PCW<br />
pressure measurements are made when the balloon of the<br />
catheter is <strong>in</strong>flated after the catheter has been properly<br />
advanced <strong>in</strong>to the pulmonary artery. The <strong>in</strong>flated balloon prevents<br />
the measurement of any pressure proximal to the balloon,<br />
and thus measurements recorded from the tip of the<br />
catheter reflect only left atrial pressure, which is commonly<br />
used as a surrogate for left ventricular end diastolic pressure.<br />
The PCW pressure trac<strong>in</strong>g should display three waveforms:<br />
the a wave represents contraction of the left atrium. The<br />
c wave is due to a rapid rise <strong>in</strong> the left ventricular pressure <strong>in</strong><br />
early systole, caus<strong>in</strong>g the mitral valve to bulge backward <strong>in</strong>to<br />
the left atrium, so that the atrial pressure <strong>in</strong>creases momentarily.<br />
The v wave is produced when blood enters the left<br />
atrium dur<strong>in</strong>g late systole, the time at which most fill<strong>in</strong>g of<br />
the left atrium occurs.<br />
Hemodynamic calculations – The follow<strong>in</strong>g formulas are<br />
used to calculate standard hemodynamic parameters derived<br />
from the above measurements:<br />
Mean* systemic arterial pressure (mBP) =<br />
diastolic BP + (systolic-diastolic BP)/3<br />
Mean* pulmonary arterial pressure (mPAP) =<br />
diastolic PAP + (systolic-diastolic PAP)/3<br />
<strong>Pulmonary</strong> vascular resistance (PVR) =<br />
(mPAP-PCW pressure)/Cardiac output (CO)<br />
<strong>Pulmonary</strong> vascular resistance <strong>in</strong>dex (PVRI) =<br />
PVR/Body surface area (BSA)<br />
Systemic vascular resistance (SVR) = (mBP-RAP)/CO<br />
Systemic vascular resistance <strong>in</strong>dex (SVRI) = SVR/BSA<br />
*Mean values may be more readily obta<strong>in</strong>ed by tak<strong>in</strong>g read<strong>in</strong>gs from<br />
bedside electronic monitor<strong>in</strong>g equipment, which obviates the need for<br />
adjust<strong>in</strong>g arithmetic means for extreme heart rates.<br />
Cardiac output measurements – There are two standard<br />
methods for determ<strong>in</strong><strong>in</strong>g cardiac output. Both methods measure<br />
pulmonary blood flow, which <strong>in</strong> the absence of an <strong>in</strong>tracardiac<br />
shunt is equal to systemic blood flow.<br />
The thermodilution method for determ<strong>in</strong><strong>in</strong>g cardiac output<br />
uses the <strong>in</strong>dicator dilution pr<strong>in</strong>ciple, where the <strong>in</strong>dicator is<br />
cold sal<strong>in</strong>e <strong>in</strong>fused as a bolus <strong>in</strong>jection <strong>in</strong>to the proximal port<br />
of the right-heart catheter. The thermistor at the distal end of<br />
the catheter then measures the appearance and disappearance<br />
of <strong>in</strong>dicator over time, and a cardiac output is then<br />
calculated. This method can be <strong>in</strong>accurate at very high or<br />
very low cardiac outputs, and can underestimate cardiac<br />
output when significant valve regurgitation is present.<br />
When us<strong>in</strong>g this technique, the cl<strong>in</strong>ician must ensure that<br />
the proximal right atrial port for <strong>in</strong>jection is actually <strong>in</strong> the<br />
right atrium, s<strong>in</strong>ce the port can be <strong>in</strong> the right ventricle when<br />
the catheter is wedged.<br />
The Fick method for determ<strong>in</strong><strong>in</strong>g cardiac output is based<br />
on the pr<strong>in</strong>ciple that consumption of a substance (oxygen <strong>in</strong><br />
this case) must equal blood flow to the organ multiplied by<br />
the difference between the arterial and venous concentrations<br />
of the substance. For this method, the formula for cardiac<br />
output is as follows:<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 17
CO =<br />
oxygen consumption per m<strong>in</strong>ute (VO 2 )<br />
(arterial oxygen content – venous oxygen content)<br />
where oxygen content is calculated as: 1.34 x [Hb] x oxygen<br />
saturation/100.<br />
In this case, the oxygen consumption can either be estimated<br />
or directly measured us<strong>in</strong>g standard techniques. 6<br />
Arterial oxygen saturation is usually determ<strong>in</strong>ed by arterial<br />
blood gas analysis, while venous oxygen saturation is determ<strong>in</strong>ed<br />
by mixed venous (pulmonary arterial) blood gas analysis.<br />
Note: In order to measure systemic arterial oxygen saturation<br />
for determ<strong>in</strong><strong>in</strong>g cardiac output us<strong>in</strong>g the Fick method,<br />
caution should be exercised when rely<strong>in</strong>g on pulse oximetry,<br />
s<strong>in</strong>ce both overestimation and underestimation can lead to<br />
significant errors <strong>in</strong> cardiac output calculations. 7,8,9<br />
Additionally, pulse oximetry may not be reliable <strong>in</strong> patients<br />
with Raynaud’s phenomenon, a common f<strong>in</strong>d<strong>in</strong>g <strong>in</strong> patients<br />
with PAH.<br />
Shunt measurements – An abnormally high pulmonary<br />
arterial saturation suggests a right-to-left shunt due to congenital<br />
heart disease and requires further evaluation and test<strong>in</strong>g<br />
to identify and quantitate the shunt. Quantitation of left-toright<br />
and/or right-to-left shunt<strong>in</strong>g is an <strong>in</strong>tegral part of rightheart<br />
catheterization. 10 However, these calculations are<br />
beyond the scope of this article.<br />
Left heart catheterization – Left-heart catheterization is<br />
not required <strong>in</strong> all patients with suspected PAH and should be<br />
reserved for patients for the follow<strong>in</strong>g diagnostic purposes:<br />
• validation of abnormal PCW pressure/evaluation of left<br />
ventricular diastolic dysfunction<br />
• suspected left-sided valvular lesion (mitral, aortic)<br />
• suspected coronary artery disease<br />
Some PAH specialists prefer to perform left-heart catheterization<br />
<strong>in</strong> all patients with suspected PAH as part of their<br />
<strong>in</strong>itial (diagnostic) evaluation, to assure that the all-exclusive<br />
workup of PAH is complete.<br />
Special Considerations<br />
Vasodilator test<strong>in</strong>g – Measurement of pulmonary vasoreactivity<br />
has an extremely important role <strong>in</strong> the diagnosis and management<br />
of patients with PAH. 11<br />
The presence of pulmonary vasoreactivity predicts the<br />
response to long-term calcium channel blocker (CCB)<br />
therapy. 12 Patients who lack pulmonary vasoreactivity respond<br />
poorly to long-term CCB use and must be treated with specific<br />
pulmonary vasodilators. Patients with significant pulmonary<br />
vasoreactivity, by contrast, have been shown to respond well<br />
to CCB therapy. Moreover, cardiac catheterization is extremely<br />
useful <strong>in</strong> these patients, with graded dos<strong>in</strong>g dur<strong>in</strong>g catheterization<br />
help<strong>in</strong>g to aid <strong>in</strong> choos<strong>in</strong>g the appropriate effective<br />
dose of CCB. 13 F<strong>in</strong>ally, pulmonary vasoreactivity has been<br />
shown to correlate with survival <strong>in</strong> patients with primary<br />
pulmonary hypertension (Figure 4). 14<br />
Caution should be exercised when consider<strong>in</strong>g vasodilator<br />
test<strong>in</strong>g and the use of a CCB for patients with PAH. Specifically,<br />
while about 25% of patients without significant heart failure<br />
symptoms exhibit pulmonary vasoreactivity, 15 patients with<br />
18 <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
Survival (percent)<br />
100<br />
75<br />
50<br />
25<br />
MR<br />
NR<br />
0<br />
0 12 24 36 48 60 72 84 96<br />
Months<br />
HR<br />
p 100 bpm<br />
• fall <strong>in</strong> heart rate to < 65/bpm with symptomatic<br />
hypotension<br />
• <strong>in</strong>tolerable side effects develop, such as headache,<br />
lightheadedness or nausea.<br />
• target response achieved (see below)<br />
• maximum dose of vasodilator agent given<br />
2. Record<strong>in</strong>g the change <strong>in</strong> hemodynamics. Focus on<br />
changes <strong>in</strong> mean pulmonary arterial pressure, PCWP, and<br />
cardiac output allows the quantitation of change <strong>in</strong> pulmonary
Powerful Medic<strong>in</strong>e.<br />
That’s what we’ve created through the comb<strong>in</strong>ation of Gentiva’s<br />
Specialty Pharmaceutical Services Division and Accredo Health,<br />
Incorporated. This alliance results <strong>in</strong> one of the country’s most<br />
extensive networks of pharmacies for the distribution of medications<br />
for pulmonary arterial hypertension.<br />
Accredo Therapeutics, Inc. utilizes pharmacist-led cl<strong>in</strong>ical teams<br />
specializ<strong>in</strong>g <strong>in</strong> PAH therapies. Our focus on pulmonary arterial<br />
hypertension results <strong>in</strong> cl<strong>in</strong>ically advanced programs and services,<br />
<strong>in</strong>clud<strong>in</strong>g home nurs<strong>in</strong>g, to support the PAH community and their<br />
physicians. Our growth has also allowed <strong>in</strong>creased access to<br />
managed care contracts mak<strong>in</strong>g it easier for patients to receive<br />
the care they need.<br />
One th<strong>in</strong>g that rema<strong>in</strong>s constant is the friendly, car<strong>in</strong>g team of<br />
professionals who will work with you day <strong>in</strong> and day out. We <strong>in</strong>vite<br />
you to call them for answers to any questions you may have<br />
concern<strong>in</strong>g PAH therapies.
Table 2—Common <strong>Pulmonary</strong> Vasodilator Agents<br />
and Their Doses<br />
Epoprostenol Adenos<strong>in</strong>e Nitric oxide<br />
Route of<br />
adm<strong>in</strong>istration Intravenous Intravenous Inhaled<br />
<strong>in</strong>fusion <strong>in</strong>fusion<br />
Systemic<br />
effect Moderate ↓ SVR Moderate ↓ SVR No ∆ SVR<br />
Use(s) Short/long-term Short term only Short/long-term<br />
Dose range 2-10 ng/ 50-500 ng/ 10 ppm<br />
kg/m<strong>in</strong> kg/m<strong>in</strong><br />
vascular resistance (see above), however close attention must<br />
be given to systemic blood pressure, heart rate, and oxygen<br />
saturation as well, to ensure patient safety dur<strong>in</strong>g up-titration<br />
of the pulmonary vasodilator.<br />
3. Interpret<strong>in</strong>g the change <strong>in</strong> hemodynamics. Some PAH<br />
experts regard a postive pulmonary vasodilator response as<br />
one <strong>in</strong> which the mean pulmonary arterial pressure falls by at<br />
least 22%, and others use a fall <strong>in</strong> pulmonary vascular resistance<br />
of at least 26% to label a positive response. There is no<br />
accepted standard for determ<strong>in</strong>ation of pulmonary vasoreac-<br />
(Dr Brundage, cont<strong>in</strong>ued from page 3)<br />
Dr Brundage’s team at UCLA had enrolled more than 300<br />
patients with pulmonary hypertension, one of the largest<br />
groups <strong>in</strong> the country at one center.<br />
Follow<strong>in</strong>g his departure from UCLA <strong>in</strong> 1998,<br />
Dr Brundage accepted the medical director’s post at the<br />
Heart Institute of the Cascades <strong>in</strong> Bend, Oregon. At that<br />
po<strong>in</strong>t he began focus<strong>in</strong>g more attention on the development<br />
and f<strong>in</strong>ancial resources of <strong>PHA</strong>. He had already<br />
become chairman of the association’s Scientific Advisory<br />
Board <strong>in</strong> 1996, a position he held until 2001. The<br />
Advisory Board has been renamed the Scientific Leadership<br />
Council. This year he agreed to become president<br />
of <strong>PHA</strong> and also serves as a member of the Board<br />
of Trustees. As a key figure <strong>in</strong> <strong>PHA</strong>’s growth, Dr Brundage<br />
has seen the association’s annual budget grow from about<br />
$100,000 to $1.8 million <strong>in</strong> only five years. Gifts from<br />
foundations, corporations, and <strong>in</strong>dividuals have spurred<br />
the group’s efforts <strong>in</strong> research and have helped it award<br />
grants to young <strong>in</strong>vestigators seek<strong>in</strong>g new therapies for<br />
PH as they pursue a career <strong>in</strong> this field.<br />
“I th<strong>in</strong>k <strong>PHA</strong> will encourage the formation of another<br />
patient registry. Many th<strong>in</strong>gs have changed s<strong>in</strong>ce the formation<br />
of the first NIH registry. We might be able to collect<br />
data that will help us determ<strong>in</strong>e which patients are<br />
candidates for lung transplantation. We may also <strong>in</strong>vestigate<br />
the appropriate use of warfar<strong>in</strong>. The current recom-<br />
20 <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
tivity. However, a consensus statement prepared for the<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Association has recently been<br />
released, 16 and data exist that correlate a robust vasodilator<br />
response with improved prognosis compared with patients<br />
without such a response.<br />
Congenital heart disease – A large number of alternative<br />
etiologies of pulmonary hypertension should be enterta<strong>in</strong>ed<br />
when evaluat<strong>in</strong>g a patient with suspected PAH. Of particular<br />
importance is the patient with congenital heart disease.<br />
Because congenital heart disease can be easily overlooked<br />
or missed, cardiac catheterization may be the only study to<br />
uncover its existence. Thus, when plann<strong>in</strong>g catheterization<br />
for PAH patients, careful consideration should be given to the<br />
measurements that are to be obta<strong>in</strong>ed at the time of catheterization.<br />
In particular, when the patient develops hypoxemia<br />
with exercise, the cl<strong>in</strong>ician should take extra care not to miss<br />
an <strong>in</strong>tracardiac shunt, which may be due to an atrial septal<br />
defect, especially of the s<strong>in</strong>us venosus type, which may be<br />
missed at echocardiography, or may be due to the presence<br />
of anomalous pulmonary ve<strong>in</strong>s. Standard measurements for<br />
patients with suspected <strong>in</strong>tracardiac shunts should always<br />
<strong>in</strong>clude blood sampl<strong>in</strong>g at various sites to determ<strong>in</strong>e oxygen<br />
saturation at all levels (SVC, IVC, RA, RV, and PA, see<br />
Measurements to Record, above).<br />
(cont<strong>in</strong>ued on next page)<br />
mendation is that all patients with pulmonary hypertension<br />
receive warfar<strong>in</strong>, but this practice has never been<br />
adequately evaluated <strong>in</strong> a randomized study. So we need<br />
to determ<strong>in</strong>e <strong>in</strong> a randomized, placebo-controlled study<br />
whether warfar<strong>in</strong> makes any difference <strong>in</strong> the long-term<br />
outcome of patients with pulmonary hypertension. It has<br />
been presumed that there is a thrombotic aspect of the<br />
disease but the long-term beneficial effect of anticoagulation<br />
has never been proven.<br />
“This new registry will have thousands of patients <strong>in</strong> it<br />
throughout the country. S<strong>in</strong>ce we have representatives on<br />
the Scientific Leadership Council from Canada, Ireland,<br />
Germany, and Italy, it could be an <strong>in</strong>ternational registry,”<br />
he added. “As president of <strong>PHA</strong>, my ma<strong>in</strong> focus will be to<br />
raise revenues <strong>in</strong> support of all of these projects. I am<br />
strongly committed to research to f<strong>in</strong>d a cure and rais<strong>in</strong>g<br />
millions of dollars <strong>in</strong> this effort. This is the best way to get<br />
to the cure. <strong>Pulmonary</strong> hypertension is multifactorial. The<br />
more we study this disease the more clear it becomes that<br />
it is a polyglot with many causes. Fortunately, many different<br />
k<strong>in</strong>ds of therapies are be<strong>in</strong>g developed.”<br />
In recognition of his achievements, <strong>PHA</strong> has given<br />
Dr Brundage its Physician of the Year Award. Widely<br />
respected <strong>in</strong> the medical community for his commitment<br />
to f<strong>in</strong>d<strong>in</strong>g a cure for pulmonary hypertension, Dr Brundage<br />
has also earned the respect of colleagues and patients<br />
alike for his compassionate attitude and the quality of<br />
patient care he provides. PH
Fig. 5—Left panel: Balloon flotation catheter record<strong>in</strong>gs of pulmonary<br />
arterial (PA) and right ventricular (RV) pressure <strong>in</strong> a patient with<br />
pulmonary hypertension. Right panel: Record<strong>in</strong>g of the same PA<br />
pressure after <strong>in</strong>flation of balloon, mistakenly labeled as pulmonary<br />
capillary wedge pressure (PCW). Note the dashed vertical l<strong>in</strong>es<br />
depict<strong>in</strong>g the peak PA and purported PCW pressure trac<strong>in</strong>gs, which<br />
occur at the same time <strong>in</strong> the cardiac cycle. Also note the lack of<br />
a and v waves <strong>in</strong> the purported PCW record<strong>in</strong>g. Measurement of<br />
arterial saturation from blood withdrawn from the distal catheter port<br />
demonstrated a saturation of 74%, with simultaneous arterial sample<br />
measured at 99%. If this had been an actual PCW record<strong>in</strong>g, oxygen<br />
saturation from the distal catheter port would also have been 99%.<br />
Also, s<strong>in</strong>ce the v waves <strong>in</strong> a true PCW record<strong>in</strong>g are transmitted<br />
waves, the peaks <strong>in</strong> v waves would have occurred later than the peak<br />
PA pressure waves. (I, AVF, V = electrocardiogram leads.) (Images<br />
courtesy of Blaufuss Multimedia Laboratories, San Francisco, CA.)<br />
Pitfalls of Measurements<br />
Incorrect record<strong>in</strong>gs of PCW. A common pitfall when measur<strong>in</strong>g<br />
PCW pressure <strong>in</strong> patients with PAH <strong>in</strong>volves <strong>in</strong>correct<br />
<strong>in</strong>terpretation. This occurs when the right-heart balloon flotation<br />
catheter is not <strong>in</strong> proper position, yield<strong>in</strong>g an <strong>in</strong>accurate<br />
pressure trac<strong>in</strong>g (Figure 5). The most common cause of this<br />
error is the record<strong>in</strong>g of a dampened pulmonary arterial pressure<br />
rather than a true occlusion pressure. This error results <strong>in</strong><br />
a falsely elevated pressure measurement, often mislead<strong>in</strong>g the<br />
cl<strong>in</strong>ician <strong>in</strong>to believ<strong>in</strong>g that the patient has pulmonary venous<br />
hypertension rather than PAH.<br />
This author frequently employs two techniques for avoid<strong>in</strong>g<br />
this measurement error:<br />
1) Partially <strong>in</strong>flat<strong>in</strong>g the balloon and gentle forward<br />
advancement of the catheter, <strong>in</strong> order to better seat and seal<br />
the catheter aga<strong>in</strong>st the walls of the pulmonary artery branch.<br />
2) Validat<strong>in</strong>g an abnormally elevated measurement by gently<br />
withdraw<strong>in</strong>g a blood sample from the distal port of the<br />
right-heart catheter dur<strong>in</strong>g balloon <strong>in</strong>flation and PCW pressure<br />
record<strong>in</strong>g, to ensure that the saturation of the sample matches<br />
systemic arterial (left atrial) saturation, ie, if the catheter is<br />
correctly placed <strong>in</strong> the wedge position, the oxygen saturation<br />
of the blood distal to the catheter should be very high (see<br />
text, Figure 5).<br />
Another problem with PCW pressure measurements is over<strong>in</strong>flation<br />
or excessive advancement of the balloon, which<br />
results <strong>in</strong> “over-wedg<strong>in</strong>g.” Prolonged over-wedg<strong>in</strong>g will yield a<br />
falsely high PCW pressure and may result <strong>in</strong> pulmonary <strong>in</strong>farction.<br />
If an accurate PCW pressure cannot be obta<strong>in</strong>ed dur<strong>in</strong>g<br />
right-heart catheterization, it is prudent to consider direct<br />
measurement of left ventricular end diastolic pressure via<br />
left-heart catheterization.<br />
Summary<br />
Cardiac catheterization should be considered for def<strong>in</strong>itive<br />
diagnosis <strong>in</strong> all patients suspected of PAH. It is the only reliable<br />
method for this purpose, it can be used for determ<strong>in</strong><strong>in</strong>g<br />
vasodilator responsiveness of the pulmonary vasculature, and<br />
if is part of a standard diagnostic workup for patients with<br />
suspected congenital heart disease. For patients with suspected<br />
secondary forms of PAH, it is especially important to be<br />
certa<strong>in</strong> that the diagnosis is accurate, as many of these<br />
patients have concomitant left heart and lung disease that<br />
could confound the diagnosis. PH<br />
References<br />
1. Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension:<br />
a national prospective study. Ann Intern Med 1987;107:216-223.<br />
2. D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival <strong>in</strong> patients with primary<br />
pulmonary hypertension. Results from a national prospective registry.<br />
Ann Intern Med 1991;115:343-9.<br />
3. Homma A, Anzueto A, Peters JI, et al. <strong>Pulmonary</strong> artery systolic pressures<br />
estimated by echocardiogram vs cardiac catheterization <strong>in</strong> patients<br />
await<strong>in</strong>g lung transplantation. J Heart Lung Transplant 2001; 20:833-9.<br />
4. Shapiro SM, Oudiz RJ, Cao T, et al. Primary pulmonary hypertension:<br />
improved long-term effects and survival with cont<strong>in</strong>uous <strong>in</strong>travenous<br />
epoprostenol <strong>in</strong>fusion. J Am Coll Cardiol 1997 30:343-9.<br />
5. Rich S, D'Alonzo GE, Dantzker DR, Levy PS. Magnitude and implications<br />
of spontaneous hemodynamic variability <strong>in</strong> primary pulmonary<br />
hypertension. Am J Cardiol 1985;55:159-63.<br />
6. Mueller HS, Chatterjee K, Davis KB, et al. ACC expert consensus document.<br />
Present use of bedside right heart catheterization <strong>in</strong> patients with<br />
cardiac disease. J Am Coll Cardiol 1998; 32:<br />
840-64.<br />
7.Benson JP, Venkatesh B, Patla V. Mislead<strong>in</strong>g <strong>in</strong>formation from pulse<br />
oximetry and the usefulness of cont<strong>in</strong>uous blood gas monitor<strong>in</strong>g <strong>in</strong> a post<br />
cardiac surgery patient. Intensive Care Med 1995; 21:437-9.<br />
8.Vicenzi MN, Gombotz H, Krenn H, Dorn C, Rehak P. Transesophageal<br />
versus surface pulse oximetry <strong>in</strong> <strong>in</strong>tensive care unit patients. Crit Care<br />
Med 2000; 28:2268-70.<br />
9.Van de Louw A, Cracco C, Cerf C, et al. Accuracy of pulse oximetry <strong>in</strong><br />
the <strong>in</strong>tensive care unit. Intensive Care Med 2001; 27):1606-13.<br />
10. Wood P. Diseases of the Heart and Circulation. 3d ed., Philadelphia,<br />
Pa: Lipp<strong>in</strong>cott; 1968.<br />
11. Rich S, Brundage BH. High-dose calcium channel-block<strong>in</strong>g therapy<br />
for primary pulmonary hypertension: evidence for long-term reduction <strong>in</strong><br />
pulmonary arterial pressure and regression of right ventricular hypertrophy.<br />
Circulation 1987;76:135-41.<br />
12: Schrader B, Inbar S, Kaufman L, et al: Comparison of the effects of<br />
adenos<strong>in</strong>e and nifedip<strong>in</strong>e <strong>in</strong> pulmonary hypertension. J Am Coll Cardiol<br />
1992;19:1060.<br />
13. Rich S, Kaufmann E. High dose titration of calcium channel block<strong>in</strong>g<br />
agents for primary pulmonary hypertension: guidel<strong>in</strong>es for short-term<br />
drug test<strong>in</strong>g. J Am Coll Cardiol 1991;18:1323-7.<br />
14. Raffy O, Azarian R, Brenot F, et al. Cl<strong>in</strong>ical significance of the pulmonary<br />
vasodilator response dur<strong>in</strong>g short-term <strong>in</strong>fusion of prostacycl<strong>in</strong> <strong>in</strong><br />
primary pulmonary hypertension. Circulation 1996;93:484-8.<br />
15. Ga<strong>in</strong>e SP, Rub<strong>in</strong> LJ. Primary pulmonary hypertension. Lancet<br />
1998;352:719-25.<br />
16. www.phassociation.org/medical<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 21
(<strong>Pulmonary</strong> <strong>Hypertension</strong> Roundtable, cont<strong>in</strong>ued from back cover)<br />
pulmonary arterial hypertension, recogniz<strong>in</strong>g that <strong>in</strong> some<br />
patients they do occur simultaneously?<br />
Dr Badesch: I would suggest look<strong>in</strong>g for physical exam f<strong>in</strong>d<strong>in</strong>gs<br />
that suggest pulmonary hypertension or pulmonary fibrosis,<br />
and then us<strong>in</strong>g echocardiography to support or ref<strong>in</strong>e the<br />
diagnosis of pulmonary hypertension. A right-heart catheterization<br />
can be done to confirm the presence of pulmonary<br />
hypertension. The diffus<strong>in</strong>g capacity can fall <strong>in</strong> either pulmonary<br />
fibrosis or pulmonary hypertension, but if it falls <strong>in</strong><br />
isolation, mean<strong>in</strong>g that the lung volumes are normal, it may<br />
suggest the presence of pulmonary hypertension.<br />
Dr McLaughl<strong>in</strong>: Would the pulmonary function test then be an<br />
appropriate screen<strong>in</strong>g tool to perform on an annual basis <strong>in</strong><br />
the scleroderma population?<br />
Dr Badesch: I th<strong>in</strong>k it is very reasonable to follow the PFTs<br />
regularly. If you see an isolated fall <strong>in</strong> the diffus<strong>in</strong>g capacity<br />
this should raise the possibility and lead to further evaluation,<br />
perhaps with an echocardiogram.<br />
Dr Seibold: The pearls are that virtually all scleroderma<br />
patients who have pulmonary arterial hypertension have a diffus<strong>in</strong>g<br />
capacity less than 55% of predicted. There are a couple<br />
of data sets that argue that when the percent of forced<br />
vital capacity (FVC) is compared with the percent of DLCO,<br />
if that ratio is elevated, it also enriches for the diagnosis of<br />
pulmonary arterial hypertension. One published series suggests<br />
a ratio of greater than 1.4. Our own data at our center<br />
suggest that that ratio might be 1.8.<br />
Dr McLaughl<strong>in</strong>: Is there a population that should have<br />
echocardiography on a regular basis?<br />
Dr Seibold: Yes. We might want to step back a step. Before<br />
I said that pulmonary arterial hypertension patients are typically<br />
dyspneic and sometimes cl<strong>in</strong>ical dyspnea on exertion is<br />
missed <strong>in</strong> rheumatologic practice. Scleroderma patients have<br />
a chronic catabolic disease; they tend to have ambulation difficulties,<br />
they tend to become very sedentary for orthopedic<br />
and musculoskeletal and peripheral vascular reasons, complicat<strong>in</strong>g<br />
their scleroderma. So they don’t typically present compla<strong>in</strong><strong>in</strong>g<br />
of dyspnea on exertion. And I th<strong>in</strong>k that rheumatologists<br />
probably as a rule tend to back <strong>in</strong>to this diagnosis<br />
through regular performance of pulmonary function test<strong>in</strong>g.<br />
And it seems quite reasonable to recommend annual pulmonary<br />
function test<strong>in</strong>g as a m<strong>in</strong>imum <strong>in</strong>terval, across the<br />
board for all scleroderma patients, probably more frequently,<br />
if you were follow<strong>in</strong>g someone early with active <strong>in</strong>flammatory<br />
fibrotic disease. It also follows that if the best screen<strong>in</strong>g test<br />
for pulmonary arterial hypertension is a Doppler echocardiogram,<br />
it is appropriate to obta<strong>in</strong> a basel<strong>in</strong>e study <strong>in</strong> all<br />
patients with scleroderma, and that this test might also be<br />
repeated at some m<strong>in</strong>imum <strong>in</strong>terval. I don’t th<strong>in</strong>k that we<br />
have the trial data that exactly validate what the standard of<br />
cl<strong>in</strong>ical practice should be. One argument should be that if<br />
22 <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
you are do<strong>in</strong>g pulmonary function test<strong>in</strong>g and you see changes<br />
<strong>in</strong> the diffus<strong>in</strong>g capacity, that might trigger repeat of the<br />
Doppler echocardiogram. Another argument might be that<br />
repeat Doppler echocardiograms might be done at about the<br />
same <strong>in</strong>terval as repeat pulmonary function tests.<br />
Dr McLaughl<strong>in</strong>: What do you do <strong>in</strong> your practice, Jim?<br />
Dr Seibold: I look at the pulmonary function test as the outcome<br />
and turn to the Doppler echo as a measure of process.<br />
We get basel<strong>in</strong>e echoes on as many patients as we can, but<br />
the decision to repeat is usually not triggered by time <strong>in</strong>terval<br />
but by some <strong>in</strong>dex of suspicion, either cl<strong>in</strong>ical dyspnea or a<br />
change <strong>in</strong> the pulmonary function test.<br />
Dr McLaughl<strong>in</strong>: And G<strong>in</strong>ny, what do you do <strong>in</strong> your practice?<br />
Dr Steen: I th<strong>in</strong>k that is exactly what I do. I probably do not<br />
repeat the PFT yearly <strong>in</strong> everyone if they have normal diffus<strong>in</strong>g<br />
capacity or only mildly decreased DLCO. On the other hand, if<br />
they already have a diffus<strong>in</strong>g capacity of 60 to 65% and they<br />
have had 10 years of disease, then follow<strong>in</strong>g PFT’s on a yearly<br />
basis would be helpful at least to detect changes that would<br />
precipitate do<strong>in</strong>g an echocardiogram. Many patients have<br />
echocardiograms that show mild pulmonary hypertension,<br />
which is <strong>in</strong> the range where the echo is difficult to <strong>in</strong>terpret.<br />
S<strong>in</strong>ce we don’t know how many of those are real pulmonary<br />
hypertension versus false positives, I th<strong>in</strong>k it is important to<br />
keep that <strong>in</strong> m<strong>in</strong>d and to proceed to catheterization when you<br />
f<strong>in</strong>d mild pulmonary hypertension rather than jump<strong>in</strong>g ahead<br />
and mak<strong>in</strong>g a diagnosis of this deadly disease. I know we all<br />
have had experiences where we have an echo that says that the<br />
pulmonary artery pressures are 40 and you get all worried and<br />
nervous and you do a cath and the results are totally normal.<br />
Dr Badesch: My impression is that follow-up and screen<strong>in</strong>g<br />
for pulmonary hypertension <strong>in</strong> the rheumatology and <strong>in</strong>ternal<br />
medic<strong>in</strong>e community are probably not as str<strong>in</strong>gent as what we<br />
have heard from Jim and G<strong>in</strong>ny. My sense is that by the time<br />
patients get referred to us for the evaluation and treatment of<br />
pulmonary hypertension they often have relatively advanced<br />
disease.<br />
Dr Seibold: I agree. I th<strong>in</strong>k there is a disconnect between<br />
the way the true scleroderma expert approaches this and the<br />
way the community rheumatologist/<strong>in</strong>ternist approaches this.<br />
Lack<strong>in</strong>g a pharmacoeconomic or costs of care study to actually<br />
validate it, I tend to come down <strong>in</strong> favor of a recommendation<br />
of a m<strong>in</strong>imum annual <strong>in</strong>terval. I agree that there are subsets<br />
of patients who don’t change much over time. But if we<br />
are look<strong>in</strong>g at a rate of transition from nonpulmonary hypertension<br />
to pulmonary hypertension of any level that may<br />
approach 5% of patients per year, that is a rather high <strong>in</strong>cidence<br />
and I th<strong>in</strong>k that would justify a blanket recommendation<br />
for annual pulmonary function test<strong>in</strong>g.<br />
Dr McLaughl<strong>in</strong>: And I th<strong>in</strong>k this is all more an issue now that<br />
we have effective therapies. Perhaps 10 or 15 years ago<br />
rheumatologists were not screen<strong>in</strong>g because frankly there was
not much to do, but I th<strong>in</strong>k all of that has changed<br />
<strong>in</strong> the current era.<br />
Dr Seibold: From a recent questionnaire that the<br />
Scleroderma Cl<strong>in</strong>ical Trials Consortium circulated<br />
to the broad community of United States and<br />
Canadian rheumatologists, it looked like echocardiography<br />
was be<strong>in</strong>g performed <strong>in</strong> the assessment<br />
of dyspnea only about 25% of the time.<br />
Dr McLaughl<strong>in</strong>: I’d like to talk about proceed<strong>in</strong>g<br />
with a heart catheterization to further evaluate<br />
patients who have pulmonary hypertension on an echocardiogram.<br />
One th<strong>in</strong>g that is always important <strong>in</strong> look<strong>in</strong>g at pulmonary<br />
hypertension patients is test<strong>in</strong>g for vasoreactivity. And<br />
the scleroderma population, at least <strong>in</strong> my experience, is very<br />
rarely vasoreactive and so I am frequently asked by rheumatologists<br />
“Why do we have to cath <strong>in</strong> the first place?” Dave, do<br />
you want to comment on that?<br />
Dr Badesch: That’s a good question. Our experience mimics<br />
yours somewhat <strong>in</strong> that I th<strong>in</strong>k the likelihood of acute vasoreactivity<br />
is lower <strong>in</strong> the population with scleroderma than <strong>in</strong><br />
primary pulmonary hypertension. I still th<strong>in</strong>k that cardiac<br />
catheterization plays an important role <strong>in</strong> evaluation of these<br />
patients. I th<strong>in</strong>k that establish<strong>in</strong>g their basel<strong>in</strong>e hemodynamics,<br />
or rul<strong>in</strong>g out the rare patient with an <strong>in</strong>tercardiac shunt<br />
or some other lesion that is contribut<strong>in</strong>g to their development<br />
of pulmonary hypertension, is important. Furthermore, <strong>in</strong><br />
patients who are fail<strong>in</strong>g despite the best available medical<br />
therapy, it may be important to repeat the cardiac catheterization<br />
to confirm that it is worsen<strong>in</strong>g of their pulmonary hypertension<br />
that is account<strong>in</strong>g for their symptoms. In that situation,<br />
compar<strong>in</strong>g the current hemodynamics to their basel<strong>in</strong>e<br />
results can be very helpful. So, I still feel that right-heart<br />
catheterization plays a role <strong>in</strong> these patients, but it may not<br />
be so much <strong>in</strong> terms of evaluat<strong>in</strong>g vasoreactivity as <strong>in</strong> establish<strong>in</strong>g<br />
a basel<strong>in</strong>e, rul<strong>in</strong>g out other contribut<strong>in</strong>g factors, and<br />
then hav<strong>in</strong>g that <strong>in</strong>formation available for future comparison.<br />
Dr McLaughl<strong>in</strong>: The other important measurement on the<br />
right-heart cath, particularly <strong>in</strong> this patient population, is<br />
wedge pressure or left ventricular end-diastolic pressure. This<br />
patient population tends to be older than the primary pulmonary<br />
hypertension population, they tend to have more concomitant<br />
illnesses, such as hypertension, and they may <strong>in</strong> fact<br />
have mild pulmonary hypertension on an echocardiogram that<br />
is really the result of systemic hypertension and left ventricular<br />
hypertrophy and elevation of LVEDP caus<strong>in</strong>g their pulmonary<br />
hypertension. So it is also crucial <strong>in</strong> secur<strong>in</strong>g the correct<br />
diagnosis and subsequently the correct treatment for<br />
these patients.<br />
Dr Seibold: If I had to make a quick list about why one should<br />
be will<strong>in</strong>g to do right- heart catheterization <strong>in</strong> scleroderma it<br />
would be 1) to confirm and to precisely quantify the diagnosis;<br />
2) to exclude the possibility of occult left ventricular<br />
diastolic failure; and 3) to exclude a component of concomitant<br />
cardiac problems. In around 20% of the caths that we<br />
In patients who are<br />
fail<strong>in</strong>g despite the best<br />
available medical<br />
therapy, it may be important<br />
to repeat the<br />
cardiac catheterization<br />
to confirm that it<br />
is worsen<strong>in</strong>g of their<br />
pulmonary hypertension<br />
that is account<strong>in</strong>g<br />
for their symptoms.<br />
do here, we frequently f<strong>in</strong>d that the aortic valvular<br />
lesions are a little bit worse than was suspected, or<br />
we f<strong>in</strong>d mitral valve pathology, or someth<strong>in</strong>g along<br />
those l<strong>in</strong>es that truly <strong>in</strong>fluences our approach to<br />
therapy. Fourth on the list would be that the echo<br />
is not a perfect test. It is relatively imprecise <strong>in</strong><br />
those that have estimated pulmonary artery systolic<br />
pressures less than 40. And there is a relatively<br />
substantial group of patients, maybe as many as<br />
20%, who lack a tricuspid jet and one cannot get<br />
a reliable estimate of pulmonary artery systolic<br />
pressure by Doppler. So, that would be the complete<br />
list. There is no question that rheumatologists are not<br />
request<strong>in</strong>g right-heart catheterizations by their consultants<br />
frequently enough.<br />
Dr McLaughl<strong>in</strong>: Why don’t we move along to those therapies?<br />
David, you were the pr<strong>in</strong>cipal <strong>in</strong>vestigator of the first trial of<br />
<strong>Pulmonary</strong> Arterial <strong>Hypertension</strong> <strong>in</strong> the Scleroderma Spectrum<br />
of Diseases with Flolan. Do you want to summarize the very<br />
impressive results of that trial for the group?<br />
Dr Badesch: As you know, prostacycl<strong>in</strong> was <strong>in</strong>itially developed<br />
for patients with primary pulmonary hypertension and we saw<br />
an improvement <strong>in</strong> exercise capacity, cardiopulmonary hemodynamics,<br />
and survival <strong>in</strong> a 12-week study. We attempted to<br />
replicate that study <strong>in</strong> the scleroderma population and what<br />
we found was that prostacycl<strong>in</strong> did <strong>in</strong> fact improve the exercise<br />
capacity and cardiopulmonary hemodynamics similarly to<br />
the way that it had <strong>in</strong> the population with primary pulmonary<br />
hypertension. We did not see a survival benefit over the threemonth<br />
course of that study, but the study was not powered to<br />
detect a survival benefit. I believe that the study of prostacycl<strong>in</strong><br />
<strong>in</strong> patients with scleroderma-associated pulmonary hypertension<br />
has led to the <strong>in</strong>clusion of such patients <strong>in</strong> the subsequent<br />
trials of therapeutic agents for pulmonary hypertension.<br />
Dr McLaughl<strong>in</strong>: It is important to po<strong>in</strong>t out what the prognosis<br />
is of scleroderma complicated by pulmonary hypertension <strong>in</strong> the<br />
absence of any treatment at all. It is a horrific survival curve.<br />
Dr Badesch: In look<strong>in</strong>g at several studies done prior to the<br />
use of prostacycl<strong>in</strong> <strong>in</strong> these patients, it appears as though the<br />
two-year survival rate was <strong>in</strong> the range of 40 to 55% or so, <strong>in</strong><br />
patients who developed pulmonary hypertension as a complication<br />
of scleroderma disease.<br />
Dr Steen: That has certa<strong>in</strong>ly been our experience. But we<br />
have to remember that previously the diagnoses have been<br />
made so late that the only time the diagnosis was made really<br />
was when patients had right-heart failure and clear-cut classic<br />
cl<strong>in</strong>ical pulmonary hypertension. Without treatment, even to<br />
survive two years for many patients was just unheard of. With<br />
the use of prostacycl<strong>in</strong>, my patients have had a much better<br />
survival rate and quality of life, even when the diagnosis is<br />
not made until the patient has right-heart failure.<br />
Dr McLaughl<strong>in</strong>: One of the problems that the scleroderma<br />
population sometimes has with prostacycl<strong>in</strong> is difficulty mix-<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 23
<strong>in</strong>g. Because many of them have severe Raynaud’s<br />
and digital ulcers and sometimes even amputations,<br />
this can be problematic. That is one of the<br />
reasons why the subcutaneous prostacycl<strong>in</strong> analogue,<br />
treprost<strong>in</strong>il, which has recently been FDAapproved,<br />
may be useful <strong>in</strong> those patients. The<br />
scleroderma patients were <strong>in</strong>cluded <strong>in</strong> the doublebl<strong>in</strong>d<br />
placebo-controlled randomized study of subcutaneous<br />
treprost<strong>in</strong>il and <strong>in</strong>deed benefited.<br />
Sometimes, however, that drug is difficult to use<br />
because of pa<strong>in</strong> at the <strong>in</strong>fusion site. Jim, you mentioned<br />
that there were three FDA-approved drugs.<br />
The third one is an oral therapy, bosentan. Would<br />
you like to comment on your experience with that<br />
so far? And perhaps even how the advent of an oral<br />
therapy has changed practice patterns that lead to<br />
earlier screen<strong>in</strong>g and diagnosis?<br />
Dr Seibold: There is no question that the logistical convenience<br />
of an oral therapy really revolutionizes the cl<strong>in</strong>ical<br />
approach. Prostacycl<strong>in</strong> is expensive, it is relatively cumbersome,<br />
it has more than a certa<strong>in</strong> level of day-to-day adverse<br />
effects that impact the quality of life. I agree with you that<br />
the adm<strong>in</strong>istration of treprost<strong>in</strong>il <strong>in</strong> the whole scheme of<br />
th<strong>in</strong>gs will be more convenient for scleroderma patients and<br />
they will be a little bit better able to handle that.<br />
Dr Badesch: The other important th<strong>in</strong>g to po<strong>in</strong>t out is that<br />
the mechanism of action of bosentan is considerably different<br />
from that of prostacycl<strong>in</strong>. Endothel<strong>in</strong> levels may be <strong>in</strong>creased<br />
<strong>in</strong> some patients with scleroderma, and us<strong>in</strong>g an endothel<strong>in</strong><br />
receptor antagonist <strong>in</strong> that situation may make particular<br />
sense, beyond even what you might expect <strong>in</strong> patients with<br />
pulmonary hypertension. So, it is particularly attractive on a<br />
theoretical basis to block endothel<strong>in</strong> <strong>in</strong> patients with scleroderma<br />
and scleroderma-related pulmonary hypertension. In a<br />
randomized and placebo-controlled study <strong>in</strong>volv<strong>in</strong>g over 200<br />
patients with primary pulmonary hypertension and pulmonary<br />
hypertension occurr<strong>in</strong>g <strong>in</strong> association with collagen vascular<br />
disease, bosentan-treated patients demonstrated better activity<br />
tolerance, as assessed by the 6-m<strong>in</strong>ute walk test, than patients<br />
receiv<strong>in</strong>g placebo. The drug seems to be relatively well<br />
tolerated although it is important to mention the side effects<br />
that have been seen to date. It can cause an elevation <strong>in</strong> liver<br />
function tests and this mandates follow<strong>in</strong>g liver function tests<br />
on a regular basis. In fact, the FDA has mandated test<strong>in</strong>g at<br />
least monthly. The drug has the potential to be teratogenic<br />
and therefore contraception is very important. It may cause<br />
male <strong>in</strong>fertility and young male patients should be <strong>in</strong>formed of<br />
that prior to beg<strong>in</strong>n<strong>in</strong>g the treatment. And f<strong>in</strong>ally, it can cause<br />
some mild anemia and at times some fluid retention.<br />
Dr McLaughl<strong>in</strong>: Those are important po<strong>in</strong>ts. Dave, would you<br />
like to speculate on the results <strong>in</strong> the scleroderma subpopulation<br />
BREATHE-1 trial, compared with the scleroderma population<br />
<strong>in</strong> your trial? Granted, it was a much smaller number <strong>in</strong><br />
the BREATHE-1 trial, but they didn’t seem to obta<strong>in</strong> as much<br />
benefit <strong>in</strong> terms of exercise tolerance over the 16 weeks of that<br />
trial as the scleroderma patients treated with <strong>in</strong>travenous<br />
24 <strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong><br />
The mechanism of<br />
action of bosentan is<br />
considerably different<br />
from that of prostacycl<strong>in</strong>.<br />
Endothel<strong>in</strong> levels<br />
may be <strong>in</strong>creased <strong>in</strong><br />
some patients with<br />
scleroderma, and<br />
us<strong>in</strong>g an endothel<strong>in</strong><br />
receptor antagonist<br />
<strong>in</strong> that situation may<br />
make particular sense,<br />
beyond even what<br />
you might expect <strong>in</strong><br />
patients with pulmonary<br />
hypertension.<br />
epoprostenol did <strong>in</strong> your trial. Any thoughts on that?<br />
Dr Badesch: The data suggest that <strong>in</strong> the study of<br />
<strong>in</strong>travenous prostacycl<strong>in</strong> <strong>in</strong> patients with scleroderma-associated<br />
pulmonary hypertension, there was<br />
both an improvement <strong>in</strong> the treatment group and<br />
a decl<strong>in</strong>e <strong>in</strong> the control group that accounted for<br />
the difference between study groups. In the<br />
BREATHE-1 study, when look<strong>in</strong>g at the subgroup<br />
of patients with scleroderma-associated pulmonary<br />
hypertension, it appears as though bosentan may<br />
have contributed to the ma<strong>in</strong>tenance of stability<br />
while patients <strong>in</strong> the placebo arm cont<strong>in</strong>ued to<br />
deteriorate. Now as you’ve mentioned, whether or<br />
not we can take away much of a message from that<br />
is a little <strong>in</strong> doubt because the relatively small number<br />
of patients with scleroderma <strong>in</strong>cluded <strong>in</strong> the BREATHE-1<br />
study. Whether or not bosentan can contribute to the same<br />
amount of symptomatic improvement or improvement <strong>in</strong> exercise<br />
capacity as prostacycl<strong>in</strong> <strong>in</strong> this population I th<strong>in</strong>k is still<br />
just little bit up <strong>in</strong> the air.<br />
Dr McLaughl<strong>in</strong>: So, the drug has been commercially available<br />
for 7 or 8 months. Jim, G<strong>in</strong>ny, would you like to share your<br />
experience with it so far?<br />
Dr Seibold: We were somewhat concerned when we saw the<br />
failure to improve <strong>in</strong> the scleroderma subset that was <strong>in</strong>corporated<br />
<strong>in</strong> the BREATHE-1 study, but suspect from our cl<strong>in</strong>ical<br />
use of the drug that that was an artifact of the relatively small<br />
sample size. We have about 65 scleroderma patients who<br />
are receiv<strong>in</strong>g bosentan currently. A large percentage of those<br />
patients, somewhere <strong>in</strong> the 80% range, have substantial,<br />
measurable, cl<strong>in</strong>ical improvement and improved exercise<br />
capacity. So we believe that more widespread use of the<br />
drug will validate that there is a positive cl<strong>in</strong>ical benefit from<br />
bosentan therapy.<br />
Dr McLaughl<strong>in</strong>: That is an important po<strong>in</strong>t. The scleroderma<br />
population made up a very small percentage of these <strong>in</strong>cluded<br />
<strong>in</strong> the BREATHE-1 trial and a much larger experience such as<br />
yours, Jim, is very important to del<strong>in</strong>eate how effective this<br />
therapy is <strong>in</strong> this subpopulation.<br />
Dr Badesch: I th<strong>in</strong>k it is important as we look toward the<br />
future to mention that we might beg<strong>in</strong> to comb<strong>in</strong>e some of<br />
these different therapies <strong>in</strong> patients with pulmonary hypertension<br />
due to scleroderma and perhaps we will be us<strong>in</strong>g some<br />
form of prostacycl<strong>in</strong> preparation <strong>in</strong> comb<strong>in</strong>ation with an<br />
endothel<strong>in</strong> receptor antagonist, a phosphodiesterase <strong>in</strong>hibitor,<br />
and perhaps oral L-arg<strong>in</strong><strong>in</strong>e or a nitric oxide donor. Multimodality<br />
therapy that mimics the way we treat systemic hypertension<br />
or cancer might have a greater likelihood of a positive<br />
effect <strong>in</strong> patients with pulmonary hypertension due to scleroderma.<br />
It is important to note that my comments <strong>in</strong> this regard<br />
are speculative, and not yet supported by cl<strong>in</strong>ical studies.<br />
Dr McLaughl<strong>in</strong>: I agree with that, David, and certa<strong>in</strong>ly comb<strong>in</strong>ation<br />
therapy is where we are go<strong>in</strong>g. The scleroderma
patients were <strong>in</strong>cluded <strong>in</strong> the BREATHE-2 trial, which looks at<br />
the comb<strong>in</strong>ation of bosentan and prostacycl<strong>in</strong> <strong>in</strong> patients with<br />
severe pulmonary hypertension. Scleroderma patients are also<br />
be<strong>in</strong>g <strong>in</strong>cluded <strong>in</strong> other cl<strong>in</strong>ical trails, specifically with the<br />
PDE5 <strong>in</strong>hibitor sildenafil and selective endothel<strong>in</strong> receptor<br />
antagonists. Dave, you also mentioned L-arg<strong>in</strong><strong>in</strong>e; there is an<br />
<strong>in</strong>ternational trial look<strong>in</strong>g at L-arg<strong>in</strong><strong>in</strong>e supplementation <strong>in</strong><br />
patients with pulmonary arterial hypertension that also<br />
<strong>in</strong>cludes the scleroderma spectrum of diseases.<br />
Dr Seibold: It should be emphasized that there is a level of<br />
scientific enthusiasm/optimism about the specificity of all<br />
these drugs <strong>in</strong> the scleroderma vascular lesion. We all recognize<br />
that scleroderma starts with vascular <strong>in</strong>jury frequently<br />
express<strong>in</strong>g as dysfunctional vascular change, ie, Raynaud’s<br />
phenomenon, but the endothelial <strong>in</strong>jury is important very early<br />
on. One consequence is dim<strong>in</strong>ished nitric oxide production.<br />
A second consequence is dim<strong>in</strong>ished prostacycl<strong>in</strong> synthase<br />
activity and lower prostacycl<strong>in</strong> levels. A third and potentially<br />
very important tissue response is <strong>in</strong>creased endothelial production<br />
of endothel<strong>in</strong>, which has vasoconstrictive effects and<br />
a variety of proliferative and pro<strong>in</strong>flammatory effects that may<br />
perpetuate and worsen the structural vascular <strong>in</strong>jury. So all<br />
of these agents that are be<strong>in</strong>g discussed, from L-arg<strong>in</strong><strong>in</strong>e<br />
through prostacycl<strong>in</strong> delivery systems through endothel<strong>in</strong><br />
antagonists may have some level of specifically address<strong>in</strong>g<br />
a key pathophysiologic derangement of scleroderma.<br />
Dr McLaughl<strong>in</strong>: We focus so much on the existence of pulmonary<br />
arterial hypertension <strong>in</strong> the scleroderma spectrum<br />
of diseases. Are there other rheumatologic diseases that are<br />
associated with pulmonary hypertension? I have seen patients<br />
with some different rheumatologic diseases, lupus, even just<br />
Sjögren’s syndrome, or polymyositis, present with pulmonary<br />
hypertension. Is that rare, or is that someth<strong>in</strong>g rheumatologists<br />
should keep their eye out for?<br />
Dr Steen: Well, certa<strong>in</strong>ly they are significantly less common<br />
than <strong>in</strong> scleroderma, but I th<strong>in</strong>k <strong>in</strong> the lupus population and<br />
the mixed connective disease population it is becom<strong>in</strong>g more<br />
and more of a problem. In the other diseases, Sjögren’s and<br />
myositis and even rheumatoid arthritis, pulmonary hypertension<br />
is well documented and we have all had these patients,<br />
but the frequency is much less.<br />
Dr McLaughl<strong>in</strong>: Dave, have you treated a number of patients<br />
with pulmonary hypertension and <strong>in</strong>terstitial lung disease?<br />
Clearly that population exists. One th<strong>in</strong>g we always worry<br />
about is the potential for a worsen<strong>in</strong>g <strong>in</strong> V-Q mismatch, and<br />
then sometimes we just tend to treat them for their pulmonary<br />
hypertension because there is noth<strong>in</strong>g else to do. I have treated<br />
a number of patients like that that and I can’t say that<br />
I have seen anyone develop worsen<strong>in</strong>g hypoxemia because<br />
of the V-Q mismatch. How about yourself?<br />
Dr Badesch: Initially, we excluded patients with more than<br />
mild <strong>in</strong>terstitial lung disease from the prostacycl<strong>in</strong> study,<br />
because of the concern that we would worsen ventilation per-<br />
fusion mismatch<strong>in</strong>g. I have cont<strong>in</strong>ued to be relatively cautious<br />
<strong>in</strong> my approach to those patients, but, as I am sure other centers<br />
have done, we have broadened the group of patients we<br />
will try to treat aggressively with prostacycl<strong>in</strong> and now bosentan.<br />
I agree with you that the worsen<strong>in</strong>g of ventilation perfusion<br />
mismatch<strong>in</strong>g is probably not as much of a problem as we<br />
expected it might be early on. I would add that <strong>in</strong> the population<br />
with both <strong>in</strong>terstitial lung disease and pulmonary hypertension,<br />
the early consideration of the possibility of lung<br />
transplantation is an important aspect of their care. Some of<br />
these patients may not prove to be good candidates for lung<br />
transplantation because of esophageal dysmotility and reflux<br />
and the risk of aspiration, but <strong>in</strong> the group of patients with<br />
both pulmonary hypertension and <strong>in</strong>terstitial lung disease, it<br />
may be particularly important to consider the possibility of<br />
lung transplantation early on. What do you th<strong>in</strong>k, Jim, do you<br />
end up referr<strong>in</strong>g those patients for a transplant evaluation?<br />
Dr Seibold: Dave, I really agree, but I can’t f<strong>in</strong>d a program<br />
that will accept my patients. The problem is that somewhere<br />
between 85% and 90% of these patients have esophageal<br />
dysmotility. So there are very few centers <strong>in</strong> the United States<br />
that are do<strong>in</strong>g s<strong>in</strong>gle lung transplantation <strong>in</strong> scleroderma at<br />
all, and there is a long list of centers that have automatically<br />
excluded scleroderma from consideration.<br />
Dr McLaughl<strong>in</strong>: Would anyone like to make any clos<strong>in</strong>g<br />
remarks before we f<strong>in</strong>ish up?<br />
Dr Badesch: I am pleased to see that patients with sclerodermarelated<br />
pulmonary hypertension are be<strong>in</strong>g <strong>in</strong>cluded <strong>in</strong> most of<br />
the studies now. And as Jim mentioned earlier, the level of enthusiasm<br />
for treat<strong>in</strong>g these patients has <strong>in</strong>creased over time.<br />
I hope that we will cont<strong>in</strong>ue to work collaboratively on cl<strong>in</strong>ical<br />
trials and toward improv<strong>in</strong>g the timely diagnosis of pulmonary<br />
hypertension and prompt <strong>in</strong>itiation of appropriate therapy.<br />
Dr Steen: I hope that <strong>in</strong> future studies we look for patients<br />
who have what I’d term pre-pulmonary hypertension, or are<br />
borderl<strong>in</strong>e, or at high risk, or whatever you want to call them,<br />
and see whether by very early aggressive treatment we might<br />
totally prevent or allay or delay the dangerous deadly consequences<br />
of this.<br />
Dr Seibold: I would just like to express my appreciation and<br />
admiration to the group of collegial, high-quality <strong>in</strong>vestigators<br />
<strong>in</strong> cardiology and pulmonary medic<strong>in</strong>e who have pushed the<br />
field of management options <strong>in</strong> pulmonary hypertension so<br />
far and so fast, and have made available so many different<br />
options for patients with scleroderma. It has been an astound<strong>in</strong>g<br />
several years of productivity.<br />
Dr McLaughl<strong>in</strong>: The one th<strong>in</strong>g I want to emphasize from our<br />
discussion is that recogniz<strong>in</strong>g these patients is critical. We<br />
now have someth<strong>in</strong>g that we can do for them. Early detection<br />
of pulmonary arterial hypertension <strong>in</strong> the scleroderma population<br />
might allow us to really have an impact on this devastat<strong>in</strong>g<br />
disease. PH<br />
<strong>Advances</strong> <strong>in</strong> <strong>Pulmonary</strong> <strong>Hypertension</strong> 25
Vallerie McLaughl<strong>in</strong>, MD<br />
James R. Seibold, MD<br />
David B. Badesch, MD<br />
Virg<strong>in</strong>ia Steen, MD<br />
<strong>Advances</strong> <strong>in</strong><br />
<strong>Pulmonary</strong> <strong>Hypertension</strong><br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Association<br />
850 Sligo Avenue, Suite 800<br />
Silver Spr<strong>in</strong>g, MD 20910<br />
<strong>Pulmonary</strong> <strong>Hypertension</strong> Roundtable<br />
Screen<strong>in</strong>g for PAH <strong>in</strong> Scleroderma:<br />
Identify<strong>in</strong>g Hallmarks of the Disease and<br />
Optimal Treatment Strategies<br />
Vallerie McLaughl<strong>in</strong>, MD, Associate Professor of<br />
Medic<strong>in</strong>e, Rush Presbyterian-St. Luke’s Medical<br />
Center, Chicago, Ill<strong>in</strong>ois, conducted this roundtable<br />
discussion. The panel <strong>in</strong>cluded James R. Seibold,<br />
MD, Professor and Director, UMDNJ Scleroderma<br />
Program. New Brunswick New Jersey; David B.<br />
Badesch, MD, Professor of Medic<strong>in</strong>e and Cl<strong>in</strong>ical<br />
Director, <strong>Pulmonary</strong> <strong>Hypertension</strong> Center, <strong>University</strong><br />
of Colorado Health Sciences Center, Denver,<br />
Colorado; and Virg<strong>in</strong>ia Steen, MD, Professor of<br />
Medic<strong>in</strong>e, Georgetown <strong>University</strong> Medical Center,<br />
Wash<strong>in</strong>gton, DC.<br />
Dr McLaughl<strong>in</strong>: What do we th<strong>in</strong>k is the true <strong>in</strong>cidence<br />
of pulmonary arterial hypertension <strong>in</strong> the<br />
scleroderma population?<br />
Dr Steen: We first have to separate the different<br />
k<strong>in</strong>ds of pulmonary hypertension that occur <strong>in</strong> scleroderma.<br />
Patients with limited scleroderma, or the<br />
CREST syndrome, as it is referred to, get primarily<br />
what we call vasculopathy or an isolated pulmonary<br />
hypertension unrelated to <strong>in</strong>terstitial fibrosis, and it<br />
can occur <strong>in</strong> the very serious deadly form <strong>in</strong> up to<br />
as many as 20% of patients. Other patients, and<br />
it’s anywhere from 10% to 30%, will have some<br />
evidence of either potential pulmonary hypertension<br />
or mild pulmonary hypertension as evidenced by abnormal<br />
f<strong>in</strong>d<strong>in</strong>gs on pulmonary function tests (PFTs)<br />
or echocardiograms. Another patient population,<br />
those with diffuse scleroderma, is more likely to<br />
have the <strong>in</strong>terstitial fibrosis and they can have pul-<br />
To order additional copies, call or contact <strong>PHA</strong> at 1-866-474-4742 or www.phassociation.org.<br />
monary hypertension related to that. And then there<br />
is the group of patients sort of <strong>in</strong> between, those<br />
who have a little bit of pulmonary fibrosis and a little<br />
bit of pulmonary hypertension, and depend<strong>in</strong>g on the<br />
nuances of their disease, one seems to predom<strong>in</strong>ate.<br />
Dr Seibold: Scleroderma generally segregates <strong>in</strong>to a<br />
rapidly evolv<strong>in</strong>g widespread form called diffuse scleroderma.<br />
These patients have a fairly high risk of<br />
hav<strong>in</strong>g an <strong>in</strong>flammatory pulmonary process that<br />
looks a lot like nonspecific <strong>in</strong>terstitial pneumonitis<br />
and the early onset of <strong>in</strong>terstitial fibrosis. These<br />
patients develop dyspnea and impaired exercise<br />
capacity that is a mixture of their established <strong>in</strong>terstitial<br />
lung disease but is cl<strong>in</strong>ically exacerbated by<br />
the evolution of the pulmonary vascular lesion. At<br />
the other end of the spectrum, you have limited<br />
scleroderma patients who seem to be relatively<br />
spared, although not completely, from this whole<br />
dynamic of <strong>in</strong>terstitial <strong>in</strong>flammation and fibrosis but<br />
who develop an isolated vasculopathy that really<br />
starts to become cl<strong>in</strong>ically relevant somewhere<br />
around the 10th year or so of disease. The educated<br />
guess here is that the pulmonary vascular or arteriolar<br />
lesion is universal, and it can progress slowly<br />
and show up as pulmonary arterial hypertension<br />
alone at later stages of limited scleroderma, but it is<br />
a cofactor <strong>in</strong> the morbidity of patients with <strong>in</strong>terstitial<br />
lung disease, <strong>in</strong>clud<strong>in</strong>g diffuse scleroderma.<br />
Dr McLaughl<strong>in</strong>: David, do you have any pearls on<br />
how one might differentiate pulmonary fibrosis from<br />
(cont<strong>in</strong>ued on page 22)<br />
BULK RATE<br />
US POSTAGE<br />
PAID<br />
Syracuse, NY<br />
Permit No. 999