Enzyme Reaction Rate - Mrs. R. Wingerden
Enzyme Reaction Rate - Mrs. R. Wingerden
Enzyme Reaction Rate - Mrs. R. Wingerden
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
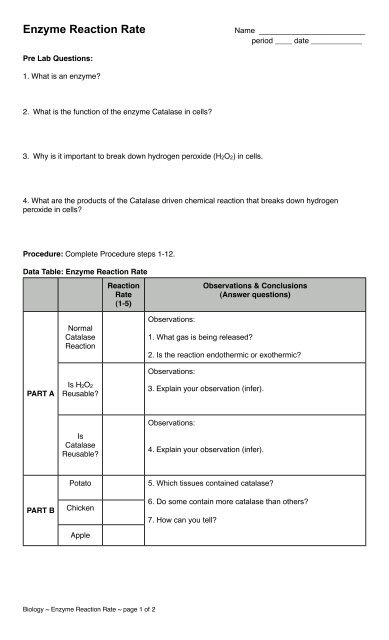
<strong>Enzyme</strong> <strong>Reaction</strong> <strong>Rate</strong><br />
Name _________________________<br />
period ____ date ____________<br />
Pre Lab Questions:<br />
1. What is an enzyme?<br />
2. What is the function of the enzyme Catalase in cells?<br />
3. Why is it important to break down hydrogen peroxide (H2O2) in cells.<br />
4. What are the products of the Catalase driven chemical reaction that breaks down hydrogen<br />
peroxide in cells?<br />
Procedure: Complete Procedure steps 1-12.<br />
Data Table: <strong>Enzyme</strong> <strong>Reaction</strong> <strong>Rate</strong><br />
<strong>Reaction</strong><br />
<strong>Rate</strong><br />
(1-5)<br />
Observations & Conclusions<br />
(Answer questions)<br />
Normal<br />
Catalase<br />
<strong>Reaction</strong><br />
Observations:<br />
1. What gas is being released?<br />
2. Is the reaction endothermic or exothermic?<br />
PART A<br />
Is H2O2<br />
Reusable?<br />
Observations:<br />
3. Explain your observation (infer).<br />
Is<br />
Catalase<br />
Reusable?<br />
Observations:<br />
4. Explain your observation (infer).<br />
PART B<br />
Potato<br />
Chicken<br />
Apple<br />
5. Which tissues contained catalase?<br />
6. Do some contain more catalase than others?<br />
7. How can you tell?<br />
Biology ~ <strong>Enzyme</strong> <strong>Reaction</strong> <strong>Rate</strong> ~ page 1 of 2
<strong>Reaction</strong><br />
<strong>Rate</strong><br />
(1-5)<br />
Observations & Conclusions<br />
(Answer questions)<br />
PART C<br />
Boiled<br />
Liver<br />
Ice Bath<br />
Liver<br />
8. You recorded the reaction rate for room temperature earlier<br />
(Normal Catalase <strong>Reaction</strong>). What is the "optimum"<br />
temperature for catalase? (This is the temperature at which the<br />
reaction proceeds fastest.)<br />
9. Why did the reaction proceed as it did at 0 ∞C?<br />
Warm<br />
Liver<br />
10. Why did the reaction proceed as it did at 100 ∞C?<br />
PART D<br />
Basic<br />
Solution<br />
pH: ____<br />
Acidic<br />
Solution<br />
pH: ____<br />
Neutral<br />
Solution<br />
pH: ____<br />
11. Does there appear to be a pH "optimum"? If so, at what<br />
pH?<br />
12. What is the effect of low or high pH on enzyme activity?<br />
Post Lab Questions:<br />
1. Summarize the general conditions necessary for effective enzyme action in this lab?<br />
2. Do you think these conditions summarized above would be the same for all enzymes? Explain.<br />
3. In Part C and Part D we changed the enzymeʼs environment and in doing so altered the rate of the<br />
reaction. Why did these manipulations effect the reaction rate?<br />
4. The liver from which we obtained catalase was dead, how then is the enzyme still active?<br />
Biology ~ <strong>Enzyme</strong> <strong>Reaction</strong> <strong>Rate</strong> ~ page 2 of 2