Guideline for Technology Transfer (Draft) - NIHS
Guideline for Technology Transfer (Draft) - NIHS
Guideline for Technology Transfer (Draft) - NIHS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
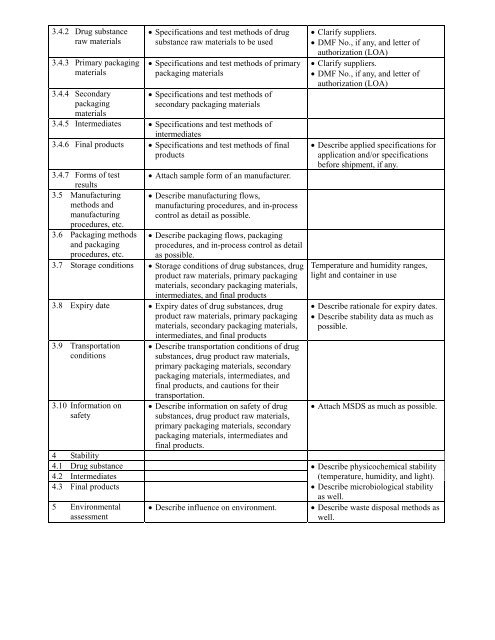
3.4.2 Drug substance<br />
raw materials<br />
3.4.3 Primary packaging<br />
materials<br />
3.4.4 Secondary<br />
packaging<br />
materials<br />
• Specifications and test methods of drug<br />
substance raw materials to be used<br />
• Specifications and test methods of primary<br />
packaging materials<br />
• Specifications and test methods of<br />
secondary packaging materials<br />
3.4.5 Intermediates • Specifications and test methods of<br />
intermediates<br />
3.4.6 Final products • Specifications and test methods of final<br />
products<br />
3.4.7 Forms of test • Attach sample <strong>for</strong>m of an manufacturer.<br />
results<br />
3.5 Manufacturing<br />
methods and<br />
manufacturing<br />
• Describe manufacturing flows,<br />
manufacturing procedures, and in-process<br />
control as detail as possible.<br />
procedures, etc.<br />
3.6 Packaging methods<br />
and packaging<br />
procedures, etc.<br />
• Describe packaging flows, packaging<br />
procedures, and in-process control as detail<br />
as possible.<br />
3.7 Storage conditions • Storage conditions of drug substances, drug<br />
product raw materials, primary packaging<br />
materials, secondary packaging materials,<br />
intermediates, and final products<br />
3.8 Expiry date • Expiry dates of drug substances, drug<br />
product raw materials, primary packaging<br />
materials, secondary packaging materials,<br />
3.9 Transportation<br />
conditions<br />
3.10 In<strong>for</strong>mation on<br />
safety<br />
4 Stability<br />
4.1 Drug substance<br />
4.2 Intermediates<br />
4.3 Final products<br />
5 Environmental<br />
assessment<br />
intermediates, and final products<br />
• Describe transportation conditions of drug<br />
substances, drug product raw materials,<br />
primary packaging materials, secondary<br />
packaging materials, intermediates, and<br />
final products, and cautions <strong>for</strong> their<br />
transportation.<br />
• Describe in<strong>for</strong>mation on safety of drug<br />
substances, drug product raw materials,<br />
primary packaging materials, secondary<br />
packaging materials, intermediates and<br />
final products.<br />
• Describe influence on environment.<br />
• Clarify suppliers.<br />
• DMF No., if any, and letter of<br />
authorization (LOA)<br />
• Clarify suppliers.<br />
• DMF No., if any, and letter of<br />
authorization (LOA)<br />
• Describe applied specifications <strong>for</strong><br />
application and/or specifications<br />
be<strong>for</strong>e shipment, if any.<br />
Temperature and humidity ranges,<br />
light and container in use<br />
• Describe rationale <strong>for</strong> expiry dates.<br />
• Describe stability data as much as<br />
possible.<br />
• Attach MSDS as much as possible.<br />
• Describe physicochemical stability<br />
(temperature, humidity, and light).<br />
• Describe microbiological stability<br />
as well.<br />
• Describe waste disposal methods as<br />
well.