Synthesis and characterization of a novel bisphthalonitrile ...
Synthesis and characterization of a novel bisphthalonitrile ...
Synthesis and characterization of a novel bisphthalonitrile ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Cao et al. – eXPRESS Polymer Letters Vol.1, No.8 (2007) 512–518<br />
2. Experimental<br />
2.1. Materials<br />
All chemicals were used as received. Paraformaldehyde<br />
(CP), toluene (99.5%), N-N-dimethylformamide<br />
(DMF, 99.5%), potassium carbonate<br />
(99%), <strong>and</strong> 1,4-dioxane (99%) were obtained from<br />
Tianjin BODI Chemicals. 4-nitrophthalonitrile<br />
(99%) was obtained from Alpha Chemical (Dezhou)<br />
Co. Ltd. 4-Aminophenol (CP) was obtained form<br />
Sinopharm Chemical Reagent Co., Ltd. Bisphenol-<br />
A was obtained form Tianjin Guangfu Fine Chemical<br />
Research Institute. All solvents used were<br />
certified A.C.S grade <strong>and</strong> used as received.<br />
2.2. <strong>Synthesis</strong><br />
2.2.1. <strong>Synthesis</strong> <strong>of</strong><br />
4-aminophenoxylphthalonitrile<br />
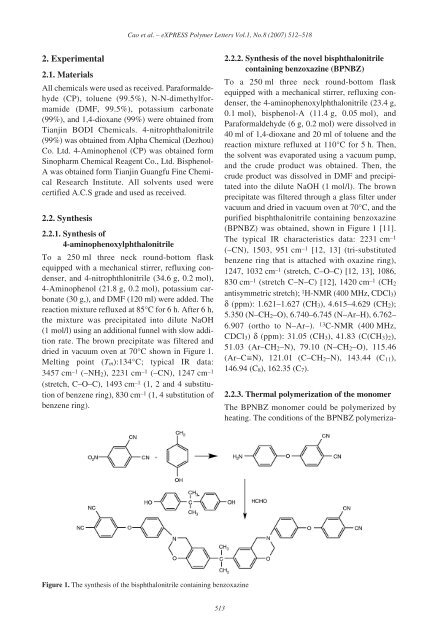
To a 250 ml three neck round-bottom flask<br />
equipped with a mechanical stirrer, refluxing condenser,<br />
<strong>and</strong> 4-nitrophthlonitrile (34.6 g, 0.2 mol),<br />
4-Aminophenol (21.8 g, 0.2 mol), potassium carbonate<br />
(30 g,), <strong>and</strong> DMF (120 ml) were added. The<br />
reaction mixture refluxed at 85°C for 6 h. After 6 h,<br />
the mixture was precipitated into dilute NaOH<br />
(1 mol/l) using an additional funnel with slow addition<br />
rate. The brown precipitate was filtered <strong>and</strong><br />
dried in vacuum oven at 70°C shown in Figure 1.<br />
Melting point (T m ):134°C; typical IR data:<br />
3457 cm –1 (–NH 2 ), 2231 cm –1 (–CN), 1247 cm –1<br />
(stretch, C–O–C), 1493 cm –1 (1, 2 <strong>and</strong> 4 substitution<br />
<strong>of</strong> benzene ring), 830 cm –1 (1, 4 substitution <strong>of</strong><br />
benzene ring).<br />
2.2.2. <strong>Synthesis</strong> <strong>of</strong> the <strong>novel</strong> <strong>bisphthalonitrile</strong><br />
containing benzoxazine (BPNBZ)<br />
To a 250 ml three neck round-bottom flask<br />
equipped with a mechanical stirrer, refluxing condenser,<br />
the 4-aminophenoxylphthalonitrile (23.4 g,<br />
0.1 mol), bisphenol-A (11.4 g, 0.05 mol), <strong>and</strong><br />
Paraformaldehyde (6 g, 0.2 mol) were dissolved in<br />
40 ml <strong>of</strong> 1,4-dioxane <strong>and</strong> 20 ml <strong>of</strong> toluene <strong>and</strong> the<br />
reaction mixture refluxed at 110°C for 5 h. Then,<br />
the solvent was evaporated using a vacuum pump,<br />
<strong>and</strong> the crude product was obtained. Then, the<br />
crude product was dissolved in DMF <strong>and</strong> precipitated<br />
into the dilute NaOH (1 mol/l). The brown<br />
precipitate was filtered through a glass filter under<br />
vacuum <strong>and</strong> dried in vacuum oven at 70°C, <strong>and</strong> the<br />
purified <strong>bisphthalonitrile</strong> containing benzoxazine<br />
(BPNBZ) was obtained, shown in Figure 1 [11].<br />
The typical IR characteristics data: 2231 cm –1<br />
(–CN), 1503, 951 cm –1 [12, 13] (tri-substituted<br />
benzene ring that is attached with oxazine ring),<br />
1247, 1032 cm –1 (stretch, C–O–C) [12, 13], 1086,<br />
830 cm –1 (stretch C–N–C) [12], 1420 cm –1 (CH 2<br />
antisymmetric stretch); 1 H-NMR (400 MHz, CDCl 3 )<br />
δ (ppm): 1.621–1.627 (CH 3 ), 4.615–4.629 (CH 2 );<br />
5.350 (N–CH 2 –O), 6.740–6.745 (N–Ar–H), 6.762–<br />
6.907 (ortho to N–Ar–). 13 C-NMR (400 MHz,<br />
CDCl 3 ) δ (ppm): 31.05 (CH 3 ), 41.83 (C(CH 3 ) 2 ),<br />
51.03 (Ar–CH 2 –N), 79.10 (N–CH 2 –O), 115.46<br />
(Ar–C≡N), 121.01 (C–CH 2 –N), 143.44 (C 11 ),<br />
146.94 (C 8 ), 162.35 (C 7 ).<br />
2.2.3. Thermal polymerization <strong>of</strong> the monomer<br />
The BPNBZ monomer could be polymerized by<br />
heating. The conditions <strong>of</strong> the BPNBZ polymeriza-<br />
Figure 1. The synthesis <strong>of</strong> the <strong>bisphthalonitrile</strong> containing benzoxazine<br />
513