Homework #1-Answers - Chemistry
Homework #1-Answers - Chemistry
Homework #1-Answers - Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Organometallic <strong>Chemistry</strong> - 4571<br />
<strong>Homework</strong> # 1: Due: February 8, 2007<br />
ANSWER KEY<br />
Check the box to the right if you want your graded homework to be placed out in the public rack outside<br />
Prof. Stanley’s office. Otherwise you will have to pick up your homework from Prof. Stanley in person:<br />
1. (38 pts) Sketch out a structure showing the geometry about the metal center as accurately as possible and<br />
clearly show the electron counting for the complexes below. Phosphine ligand abbreviations are defined in<br />
your notes (phosphine ligand section). Most molecules (some simplified) are from Organometallics, 2003, # 1<br />
& 2, from the Chem Library or on-line at the Library E-Journal site (only accessable from a campus computer).<br />
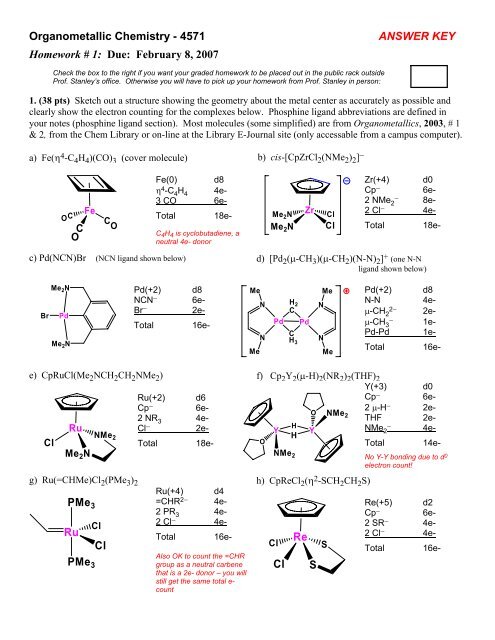
a) Fe(η 4 -C 4 H 4 )(CO) 3 (cover molecule) b) cis-[CpZrCl 2 (NMe 2 ) 2 ] −<br />
OC<br />
C<br />
O<br />
Fe<br />
C<br />
O<br />
Fe(0) d8<br />
η 4 -C 4 H 4 4e-<br />
3 CO 6e-<br />
Total 18e-<br />
C 4 H 4 is cyclobutadiene, a<br />
neutral 4e- donor<br />
Me 2 N<br />
Me 2 N<br />
Zr<br />
Cl<br />
Cl<br />
Zr(+4) d0<br />
Cp − 6e-<br />
2 NMe − 2 8e-<br />
2 Cl − 4e-<br />
Total 18e-<br />
c) Pd(NCN)Br (NCN ligand shown below) d) [Pd 2 (μ-CH 3 )(μ-CH 2 )(N-N) 2 ] + (one N-N<br />
ligand shown below)<br />
Me 2 N<br />
Br Pd<br />
Me 2 N<br />
Pd(+2) d8<br />
NCN − 6e-<br />
Br − 2e-<br />
Total 16e-<br />
Me<br />
N<br />
N<br />
Me<br />
Pd<br />
H 2<br />
C<br />
Pd<br />
C<br />
H 3<br />
Me<br />
N<br />
N<br />
Me<br />
Pd(+2) d8<br />
N-N 4eμ-CH<br />
2− 2 2eμ-CH<br />
− 3 1e-<br />
Pd-Pd 1e-<br />
Total 16e-<br />
e) CpRuCl(Me 2 NCH 2 CH 2 NMe 2 ) f) Cp 2 Y 2 (μ-H) 2 (NR 2 ) 2 (THF) 2<br />
Y(+3) d0<br />
Ru(+2) d6<br />
Cp − 6e-<br />
Cp − 6e-<br />
2 μ-H<br />
O NMe − 2e-<br />
2 NR 3 4e-<br />
2<br />
THF 2e-<br />
Ru<br />
Cl − 2e-<br />
H<br />
Y Y<br />
NMe − 2 4e-<br />
NMe 2 H<br />
Cl<br />
Total 18e-<br />
O<br />
Total 14e-<br />
Me 2 N<br />
NMe 2<br />
g) Ru(=CHMe)Cl 2 (PMe 3 ) 2 h) CpReCl 2 (η 2 -SCH 2 CH 2 S)<br />
Ru(+4) d4<br />
PMe 3<br />
Ru<br />
Cl<br />
Cl<br />
=CHR 2− 4e-<br />
2 PR 3 4e-<br />
2 Cl − 4e-<br />
Total 16e-<br />
Also OK to count the =CHR<br />
group as a neutral carbene<br />
that is a 2e- donor – you will<br />
still get the same total e-<br />
count<br />
Cl<br />
Cl<br />
Re<br />
S<br />
S<br />
No Y-Y bonding due to d 0<br />
electron count!<br />
Re(+5) d2<br />
Cp − 6e-<br />
2 SR − 4e-<br />
2 Cl − 4e-<br />
Total 16e-
HW # 1 – 2007 2<br />
i) [CpFe(C 6 H 6 )] + j) Cp 2 Nb(CH 3 )(CH 2 =CH 2 )<br />
Fe<br />
Fe(+2) d6<br />
C 6 H 6 6e-<br />
Cp − 6e-<br />
Total 18e- Nb CH 3<br />
Nb(+3) d2<br />
2 Cp − 12e-<br />
H 2 C=CH 2 2e-<br />
CH − 3 2e-<br />
Total 18e-<br />
k) CpOs(H)(Cl)(SiMe 3 )(PR 3 ) l) NiPh(PPh 3 )(N~O) N~O ligand shown below<br />
H<br />
Os<br />
PR 3<br />
Os(+4) d4<br />
Cp − 6e-<br />
SiR − 3 2e-<br />
H- 2e-<br />
PR 3 2e-<br />
Cl − 2e-<br />
R<br />
Ph 3 P Ni(+2) d8<br />
N~O − 4e-<br />
Ni<br />
N<br />
PPh 3 2e-<br />
Ph<br />
O<br />
− 2e-<br />
Total 16e-<br />
Cl SiMe 3<br />
Total 18e-<br />
m) cis-Re(CO) 3 (bipy)(NR 2 ) bipy = bipyridine n) [Ru(C 6 H 6 )(C~N)(NCCH 3 ] + C~N below<br />
OC<br />
OC<br />
N<br />
Re<br />
C<br />
O<br />
NR 2<br />
N<br />
Re(+1) d6<br />
Ru(+2) d6<br />
NR − 2 2e-<br />
C~N − 4ebipy<br />
4e-<br />
C<br />
Ru<br />
6 H 6 6e-<br />
3 CO 6e-<br />
N C<br />
NCCH 3 2e-<br />
CH3<br />
Total 18e- NMe 2 Total 18e-<br />
o) Cp 2 Fe 2 (μ-C≡C)(CO) 4 p) [Cp 2 Fe] +<br />
O<br />
C<br />
Fe<br />
C<br />
O<br />
O<br />
C<br />
Fe<br />
O<br />
C<br />
Fe(+2) d6<br />
Cp − 6eμ-C≡C<br />
2− 2e-<br />
2 CO 4e-<br />
Total 18e-<br />
Fe<br />
Fe(+3) d5<br />
2 Cp − 12e-<br />
Total 17e-<br />
q) (C 6 H 6 )Cr(CO) 3 r) Co 2 (μ-CO) 2 (CO) 6<br />
OC<br />
C<br />
O<br />
Cr<br />
C<br />
O<br />
Cr(0) d6<br />
C 6 H 6 6e-<br />
3 CO 6e-<br />
Total 18e-<br />
O<br />
C O<br />
C<br />
C<br />
O Co(0) d9<br />
2 μ-CO 2e-<br />
Co Co<br />
C<br />
C<br />
O 3 CO 6e-<br />
O<br />
1 Co-Co 1e-<br />
OC<br />
C<br />
O<br />
C<br />
O<br />
Total 18e-
HW # 1 – 2007 3<br />
2. (12 pts) Propose an 18e- structure for the following metal/ligand combinations. Use at least one of each<br />
metal and ligand listed. Complexes must be neutral. Don’t use more than 2 metal centers. Clearly show your<br />
electron counting. Ligands are shown without charges, please indicate the proper ligand charge in your electron<br />
counting. Draw a reasonable structure showing the geometry about the metal center(s).<br />
a) Tc, Cp, O, P(OMe) 3 b) Ni, dmpe (chelating), μ-CO, CO<br />
O<br />
Tc<br />
P(OMe) 3 Total 18e-<br />
Tc(+3) d4<br />
Cp − 6e-<br />
O 2− 4e-<br />
2 PR 3 4e-<br />
P(OMe) 3<br />
O<br />
C O<br />
C<br />
C<br />
O Ni(0) d10<br />
2 μ-CO 2e-<br />
Ni Ni<br />
CO 2e-<br />
C<br />
PR 2 O R 2 P<br />
2 PR 3 (dmpe) 4e-<br />
Total 18e-<br />
R 2 P PR 2<br />
c) Ta, Cp, C-R d) Cr, Cl, C≡NMe<br />
Ta C R<br />
Ta(+5) d0<br />
2 Cp − 12e-<br />
CR 3− 6e-<br />
Total 18e-<br />
RN<br />
RN<br />
Cl<br />
C<br />
C<br />
Cr<br />
C<br />
N<br />
R<br />
Cl<br />
C<br />
C<br />
NR<br />
NR<br />
Cr(+2) d4<br />
2 Cl − 4e-<br />
5 C≡NR 10e-<br />
Total 18e-<br />
7-coordinate Cr with<br />
“skinny” ligands is A-OK