Lecture Summary 11 September 20, 2004 - Cook Group
Lecture Summary 11 September 20, 2004 - Cook Group
Lecture Summary 11 September 20, 2004 - Cook Group
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Lecture</strong> <strong>Summary</strong> <strong>11</strong><br />
<strong>September</strong> <strong>20</strong>, <strong>20</strong>04<br />
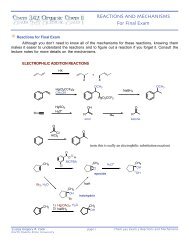
Chapter 4 - Stereochemistry of Alkanes and Cycloalkanes<br />
Conformations of Cyclopropane<br />
Cyclopropane has a high degree of angle strain due to the highly distorted bond angles. The angle<br />
between the carbons is 60°. This cannot be accommodated by sp 3 -hybridized atoms. Thus, the<br />
bonds actually are bent sigma bonds.<br />
60°<br />
Cyclopropane only has three atoms in the ring, so it must be a flat structure. Indeed, it is the only<br />
cycloalkane that is completely flat. This causes a high degree of torsional strain because all the<br />
bonds are eclipsed.<br />
Conformations of Cyclobutane<br />
Cyclobutane is almost as high in ring strain energy as cyclopropane, but being a bigger ring, it has<br />
just enough flexibility to pucker slightly. This helps to alleviate a little bit of the torsional strain. The<br />
bonds are not quite eclipsed.<br />
©<strong>20</strong>04 Gregory R. <strong>Cook</strong> page 1 Chem 341<br />
North Dakota State University
Conformations of Cyclopentane<br />
Cyclopentane has even more flexibility and can bend more. This removes a large amount of the<br />
angle strain and the torsional strain. Note that at any given time cyclopentane has some bonds<br />
more eclipsed than others.<br />
Conformations of Cyclohexane<br />
Cyclohexane has no ring strain. In the chair conformation, all the bonds are staggered and all the<br />
angles are ideal. Note that the bonds that point straight up or down from the plane of the ring are<br />
in axial positions and the bonds that point out away from the plane of the ring are called equatorial<br />
positions.<br />
Cyclohexanes are flexible enough to undergo a ring flip from one chair conformation to another at<br />
room temperature (and below). When the chair flips, all the equatorial positions become axial and<br />
all the axial positions become equatorial.<br />
©<strong>20</strong>04 Gregory R. <strong>Cook</strong> page 2 Chem 341<br />
North Dakota State University
If the cyclohexane ring is substituted with subsituents like a methyl group, the two chair<br />
conformations are no longer equal in energy (like the all H cyclohexane). The conformation with<br />
the methyl group in the axial position is higher in energy because the methyl group has steric<br />
interactions with the other axial groups (1,3-diaxial interactions).<br />
1,3-diaxial interaction<br />
axial methyl<br />
H<br />
H<br />
H<br />
H<br />
CH 3<br />
H<br />
ring flip<br />
H<br />
H<br />
H<br />
H<br />
H<br />
equatorial methyl<br />
CH 3<br />
1.8 kcal/mol more<br />
stable conformation<br />
axial methylcyclohexane side and top views<br />
equatorial methylcyclohexane side and top views<br />
©<strong>20</strong>04 Gregory R. <strong>Cook</strong> page 3 Chem 341<br />
North Dakota State University