Lecture Summary 18 October 8, 2004 - Cook Group
Lecture Summary 18 October 8, 2004 - Cook Group
Lecture Summary 18 October 8, 2004 - Cook Group
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 6 - Alkenes: Structure and Reactivity<br />
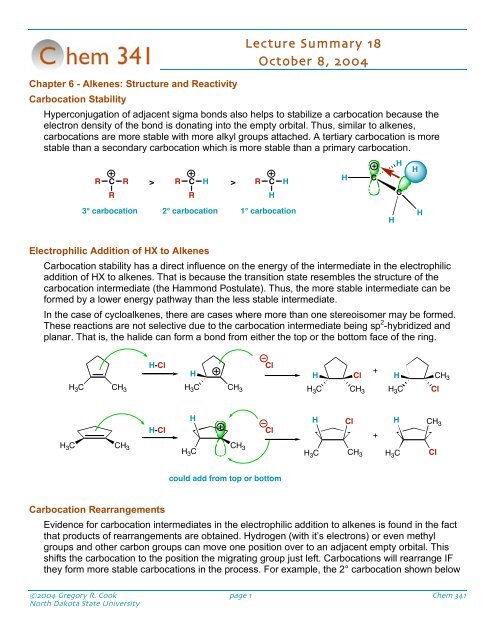
Carbocation Stability<br />
<strong>Lecture</strong> <strong>Summary</strong> <strong>18</strong><br />
<strong>October</strong> 8, <strong>2004</strong><br />
Hyperconjugation of adjacent sigma bonds also helps to stabilize a carbocation because the<br />
electron density of the bond is donating into the empty orbital. Thus, similar to alkenes,<br />
carbocations are more stable with more alkyl groups attached. A tertiary carbocation is more<br />
stable than a secondary carbocation which is more stable than a primary carbocation.<br />
R<br />
C<br />
R<br />
R<br />
> R C H ><br />
R<br />
R<br />
C<br />
H<br />
H<br />
H<br />
C<br />
H<br />
C<br />
H<br />
3° carbocation 2° carbocation 1° carbocation<br />
H<br />
H<br />
Electrophilic Addition of HX to Alkenes<br />
Carbocation stability has a direct influence on the energy of the intermediate in the electrophilic<br />
addition of HX to alkenes. That is because the transition state resembles the structure of the<br />
carbocation intermediate (the Hammond Postulate). Thus, the more stable intermediate can be<br />
formed by a lower energy pathway than the less stable intermediate.<br />
In the case of cycloalkenes, there are cases where more than one stereoisomer may be formed.<br />
These reactions are not selective due to the carbocation intermediate being sp 2 -hybridized and<br />
planar. That is, the halide can form a bond from either the top or the bottom face of the ring.<br />
H-Cl<br />
H<br />
Cl<br />
H<br />
Cl<br />
+<br />
H<br />
CH 3<br />
H 3 C CH 3<br />
H 3 C CH 3<br />
H 3 C<br />
Cl<br />
H-Cl<br />
H<br />
Cl<br />
H<br />
Cl<br />
+<br />
H CH 3<br />
H 3 C CH 3<br />
H 3 C<br />
H 3 C CH 3<br />
CH 3<br />
H 3 C CH 3<br />
H 3 C<br />
Cl<br />
could add from top or bottom<br />
Carbocation Rearrangements<br />
Evidence for carbocation intermediates in the electrophilic addition to alkenes is found in the fact<br />
that products of rearrangements are obtained. Hydrogen (with it’s electrons) or even methyl<br />
groups and other carbon groups can move one position over to an adjacent empty orbital. This<br />
shifts the carbocation to the position the migrating group just left. Carbocations will rearrange IF<br />
they form more stable carbocations in the process. For example, the 2° carbocation shown below<br />
©<strong>2004</strong> Gregory R. <strong>Cook</strong> page 1 Chem 341<br />
North Dakota State University
will rearrange to the more stable 3° carbocation by shifting a hydrogen over one position. In the<br />
reaction of 2-methyl-1-butene with HCl, a mixture of products is obtained.<br />
H 3 C<br />
H<br />
C<br />
CH 3<br />
CH CH 2<br />
H-Cl<br />
protonate to form<br />
the 2° carbocation<br />
H 3 C<br />
H H<br />
C CH CH 2<br />
CH 3<br />
Cl<br />
rearrange to form<br />
more stable 3°<br />
carbocation<br />
H 3 C<br />
H H<br />
C CH CH 2<br />
CH 3<br />
Cl<br />
H<br />
Cl<br />
H<br />
Cl<br />
H<br />
H<br />
H 3 C<br />
C<br />
CH 3<br />
CH CH 2<br />
H 3 C<br />
C<br />
CH 3<br />
CH CH 2<br />
Chapter 7 - Alkenes: Reactions and Synthesis<br />
Preparation of Alkenes<br />
Alkenes are most commonly prepared by elimination reactions. Note that these are the opposite of<br />
additions reactions. For example, a halogenated alkane will undergo a dehydrohalogenation<br />
reaction in the presence of a good base. Alcohols, in the presence of a strong acid, will also<br />
undergo a stepwise elimination of water (dehydration) to produce an alkene.<br />
Cl<br />
K + OH -<br />
+ KCl + H 2 O<br />
H<br />
H<br />
OH<br />
H 3 PO 4<br />
O<br />
H<br />
-H2 O<br />
H<br />
H<br />
H<br />
H 2 PO 4<br />
Electrophilic Addition of X 2 to Alkenes<br />
Alkenes will react with many different electrophiles. Molecular bromine, chlorine or iodine is no<br />
different. You can think of these reagents as equivalent to an X + and an X - . Unlike the addition of<br />
HX, the halogens have the ability to share one of their lone pairs to form a bridged halonium<br />
intermediate. This is more stable than a carbocation intermediate. The result of this is that one<br />
face of a ring is blocked, so the second halide addition step can only occur trans to the first<br />
halogen.<br />
Br-Br<br />
A bromonium<br />
Br<br />
Br<br />
Br<br />
Br<br />
Bromide can only come from the<br />
bottom and kick off the bromine on<br />
the top. This reaction forms the<br />
trans product selectively.<br />
©<strong>2004</strong> Gregory R. <strong>Cook</strong> page 2 Chem 341<br />
North Dakota State University