2010 CHE 230 Chapter 9 SOLUTIONS - Illinois State University
2010 CHE 230 Chapter 9 SOLUTIONS - Illinois State University
2010 CHE 230 Chapter 9 SOLUTIONS - Illinois State University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
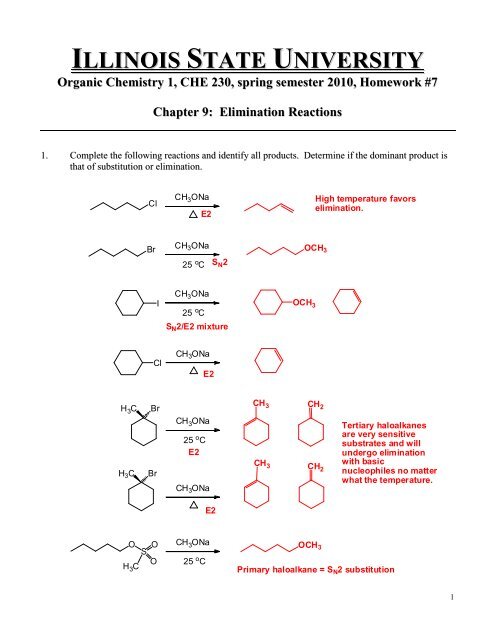
ILLINOIS STATE UNIVERSITY<br />
Organic Chemistry 1, <strong>CHE</strong> <strong>230</strong>, spring semester <strong>2010</strong>, Homework #7<br />
<strong>Chapter</strong> 9: Elimination Reactions<br />
1. Complete the following reactions and identify all products. Determine if the dominant product is<br />
that of substitution or elimination.<br />
Cl<br />
CH 3 ONa<br />
E2<br />
High temperature favors<br />
elimination.<br />
Br<br />
CH 3 ONa<br />
25 o C<br />
S N 2<br />
OCH 3<br />
I<br />
CH 3 ONa<br />
25 o C<br />
S N 2/E2 mixture<br />
OCH 3<br />
Cl<br />
CH 3 ONa<br />
E2<br />
H 3 C<br />
H 3 C<br />
Br<br />
Br<br />
CH 3 ONa<br />
25 o C<br />
E2<br />
CH 3 ONa<br />
CH 3 CH 2<br />
Tertiary haloalkanes<br />
are very sensitive<br />
substrates and will<br />
undergo elimination<br />
CH 3<br />
with basic<br />
CH 2 nucleophiles no matter<br />
what the temperature.<br />
E2<br />
O<br />
S<br />
O<br />
H 3 C<br />
O<br />
CH 3 ONa<br />
25 o C<br />
OCH 3<br />
Primary haloalkane = S N 2 substitution<br />
1
2. Complete the following reactions and identify all products. Determine if the dominant product is<br />
that of substitution (S N 2 or S N 1) or elimination.<br />
Cl<br />
H<br />
CH 3<br />
CH 2 CH 3<br />
NaN 3<br />
dmf<br />
S N 2<br />
H 3 C<br />
H<br />
N 3<br />
CH 2 CH 3<br />
Br<br />
CH 3<br />
CH 3<br />
CH 3<br />
E1 is minor<br />
KCN<br />
dmf<br />
S N 1<br />
NC<br />
CH 3<br />
CH 3<br />
CH 3<br />
H 3 C<br />
CH 3<br />
CH 3<br />
CN<br />
CH 2<br />
CH 3<br />
NaOCH 3<br />
OH<br />
Br<br />
CH 3<br />
CH 3<br />
CH 3<br />
E2<br />
H 3 C<br />
O<br />
O<br />
CH 3<br />
O<br />
I<br />
CH 3<br />
CH 3<br />
CH 3<br />
S N 1<br />
E1 is minor<br />
Ph<br />
O<br />
CH 3<br />
CH 3<br />
CH 3<br />
H 3 C<br />
CH 3<br />
O<br />
Ph<br />
H<br />
H<br />
Br<br />
CH 3<br />
CH 2 CH 3<br />
LiSCH 2 CH 3<br />
dmf<br />
S N 2<br />
H 3 C<br />
CH 2 CH 3<br />
SCH 2 CH 3<br />
Cl<br />
CH 3<br />
CH 2 CH 3<br />
CH 3 CH 2 OH<br />
S N 1-racemization<br />
E1 is minor<br />
OCH 2 CH OCH 3<br />
2 CH 3<br />
CH 3 H 3 C<br />
CH 2 CH 3 CH 2 CH 3<br />
Br<br />
H<br />
CH 3<br />
CH 2 CH 3<br />
NaOCH 3<br />
100 o C<br />
E2<br />
H<br />
H 3 C<br />
C<br />
CH 3<br />
C<br />
H<br />
Br<br />
H<br />
CH 3<br />
CH 2 CH 3<br />
KOC(CH 3 ) 3<br />
25 o C<br />
H<br />
C<br />
H 3 C<br />
CH 3<br />
C<br />
H<br />
E2-For secondary haloalkanes large bulky bases will<br />
trigger the E2 reaction no matter what the temperature is.<br />
2
3. Provide the products of the following transformation. Also state if the reaction is S N 2, S N 1, E1, or<br />
E2.<br />
Br<br />
Na OH<br />
S 25 o C<br />
N 2 + E2<br />
O<br />
O K<br />
Cl<br />
dmf<br />
S N 2 + E2<br />
CH 3 CH 2 S Li<br />
OMs nitromethane<br />
S<br />
solvent<br />
N 1 + E1<br />
MS = mesylate, a good leaving group<br />
OH<br />
H<br />
CH 3<br />
O<br />
O<br />
SCH 2 CH 3<br />
CH 2<br />
CH 2<br />
CH 3<br />
4. Provide the products of the following transformation. If there is no reaction then write NR. If<br />
there is stereochemistry, you must write out the proper answer involving stereochemistry.<br />
CH 3<br />
Br<br />
H<br />
CH 2 CH 3<br />
Na CN<br />
S N 2<br />
CH 3<br />
H<br />
CN<br />
CH 2 CH 3<br />
H<br />
Na OCH 3<br />
CH 3 CH 2 CH 3<br />
There is no reaction. There is no leaving<br />
group present.<br />
H<br />
T his is also wr itten as NaOCH 3.<br />
Br<br />
OH<br />
OCH(CH 3 ) 2<br />
CH 2 CH 3<br />
CH 3<br />
CH 2 CH 3<br />
S N 1 + E1<br />
CH 3<br />
CH 3<br />
OCH(CH 3 ) 2<br />
CH 2 CH 3<br />
H 2 C C CH 2 CH 3 CH 3 C CHCH 3<br />
CH 3 C CH 2 CH 3<br />
3
5. Complete the following reactions. You must indicate stereochemistry. If there is no reaction, then<br />
write NR.<br />
CH 3<br />
Br<br />
H<br />
CH 2 CH 3<br />
K<br />
S N 2<br />
C<br />
N<br />
CH 3<br />
H<br />
CN<br />
CH 2 CH 3<br />
O<br />
OH<br />
O<br />
O<br />
H<br />
Cl<br />
S N 1 + E1<br />
H<br />
O<br />
Ph<br />
Ph<br />
O CH 3<br />
H<br />
I<br />
CH 3<br />
CH 2 CH 3<br />
H<br />
S<br />
Li<br />
CH 3<br />
CH 2 CH 3<br />
CH 2<br />
CH 3<br />
H<br />
H<br />
S N 2<br />
H<br />
S<br />
6. Complete the following reactions. If there is no reaction, then write NR. If there is any<br />
stereochemistry, then you must address it.<br />
H<br />
Br<br />
CH 3<br />
CH 2 CH 3<br />
Br<br />
CH 3 CH 2 OH<br />
H<br />
S N 1-E1<br />
Et = -CH 2 CH 3 (ethyl)<br />
OEt<br />
CH 3<br />
CH 2 CH 3<br />
OCH 3<br />
CH 2 CH 3<br />
OEt<br />
H<br />
CH 2<br />
H CH 3 CH 2 CH 3<br />
H<br />
CH 3<br />
CHCH 3<br />
NaOCH 3<br />
25 o C<br />
S N 2-E2 mix<br />
Cl<br />
NaOCH 3<br />
100 o C<br />
E2 is dominant<br />
Br<br />
KSCH 2 CH 3<br />
dmf<br />
S N 2 is dominant<br />
SCH 2 CH 3<br />
4
7. Complete the following reactions by drawing the major and minor products. If there is no<br />
reaction, then write NR. If there any stereochemistry, then you must address it.<br />
Br<br />
CH 3<br />
CH 3<br />
CH 2<br />
KOH<br />
H 2 O<br />
E2 elimination due to a<br />
basic nucleophile<br />
Et<br />
Et<br />
Cl<br />
NaN 3<br />
dmf<br />
N 3<br />
S N 2-inversion of configuration<br />
KOt-Bu<br />
Br<br />
E2 elimination due to a<br />
basic nucleophile<br />
t-BuOH<br />
100 o C<br />
Cl<br />
CH 3<br />
CH 3<br />
CH 2<br />
NaOCH 2 CH 3<br />
E2 elimination due to a<br />
basic nucleophile<br />
CH 3 CH 2 OH<br />
100 o C<br />
5
8. Complete the following reactions. If there is no reaction, then write NR. If there is any<br />
stereochemistry, then you must address it.<br />
Cl<br />
H<br />
CH 3<br />
CH 2 CH 3<br />
NaN 3<br />
dmf<br />
S N 2<br />
H 3 C<br />
H<br />
N 3<br />
CH 2 CH 3<br />
OH<br />
CH 2 CH 3<br />
Br CH 3<br />
H<br />
F CH 3<br />
CH 2 CH 3<br />
CH 3<br />
H CH 2 CH 3<br />
OH<br />
CH 3<br />
CH 3 N<br />
Cl<br />
CH 3<br />
Br<br />
CH 3<br />
NaCl<br />
dmso<br />
H 2 O<br />
S N 1 + E1<br />
K O CH 3<br />
KCN<br />
dmf<br />
O<br />
1. CH 3 SO 2 Cl, base<br />
2. KSCH 2 CH(CH 3 ) 2<br />
S N 2<br />
HO<br />
O<br />
H<br />
S N 1 + E1<br />
NaOH, cold temp.<br />
E2<br />
No Reaction. Alcohols are not<br />
reasonable leaviing groups.<br />
HO<br />
CH 2 CH 3<br />
CH 3<br />
H 3 C<br />
CH 2 CH 3<br />
OH<br />
CHCH 3<br />
CH 2 CH 3<br />
CH 3<br />
CH 2<br />
CH 2 CH 3<br />
CH 3<br />
No Reaction. There is no leaving group.<br />
No reaction. There is no reasoable leaving group.<br />
The carbon-fluorine bond is extraordinarily strong.<br />
CH 3<br />
H<br />
N<br />
CH 3<br />
CH 2 CH 3<br />
OSO 2 CH 3<br />
O<br />
CH 2<br />
C<br />
CH 3<br />
H<br />
SCH 2 CH(CH 3 ) 2<br />
CH 3<br />
CH 2 CH 3<br />
CH 3 CH 3 N<br />
CH 3<br />
O<br />
O<br />
O<br />
H CH 3 N CH 3<br />
H<br />
CH 3 N CH 2<br />
Cl<br />
I<br />
one equivalent of<br />
NaOAc<br />
Cl<br />
sodium acetate<br />
S N 2-iodo system is more reactive.<br />
O<br />
O<br />
CH 3<br />
Cl<br />
C C<br />
S N 2<br />
Li<br />
C C<br />
6
9. Rank the following alkenes in terms of their relative stabilities.<br />
A B C D<br />
B > A > D ≈ C. B is the most stable because it is tetra-substituted. D and C are nearly equivalent because they<br />
are both di-substituted alkenes.<br />
10. Provide a mechanistic explanation for the following sequence of reaction.<br />
O<br />
D<br />
O<br />
S<br />
O<br />
CF 3<br />
D<br />
O<br />
H<br />
O<br />
S<br />
O<br />
CF 3<br />
CH 3 OH<br />
CH 3 O<br />
The triflate group is a powerful leaving group that is considered<br />
to be superior to the halogens in terms of leaving group ability.<br />
7
11. Provide a mechanistic explanation for the following sequence of reaction.<br />
CH 3<br />
O<br />
S<br />
O<br />
The Hofmann base, potassium tert -butoxide is a large base that<br />
removes the most accessible proton to induce the elimination.<br />
D<br />
O<br />
C<br />
H<br />
H<br />
D<br />
C<br />
C<br />
H<br />
CH 3<br />
+ (CH 3 ) 3 CO-H<br />
H<br />
O<br />
CH 3<br />
CH 3<br />
CH 3<br />
H<br />
O<br />
S<br />
O<br />
O<br />
8
12. Complete the following reactions. Indicate which product is the major product and which is the minor. <strong>State</strong> the<br />
pathway of elimination, either Zaitsev or Hoffmann. What is the dominant pathway in each case<br />
Br CH 3<br />
NaOCH 3 in CH 3 OH<br />
CH 3<br />
CH 2<br />
100 o C<br />
Zaitsev<br />
major<br />
minor<br />
Br<br />
KOC(CH 3 ) 3<br />
(CH 3 ) 3 COH<br />
100 o C<br />
Hofmann<br />
major<br />
cis + trans (minor)<br />
CH 2 CH 3<br />
CH 2 CH 3 CH2 CH 3<br />
Cl<br />
LiOCH 3 in CH 3 OH<br />
100 o C<br />
Zaitsev<br />
major<br />
minor<br />
CH 2 CH 2 I<br />
KOEt in EtOH<br />
EtOH = CH 3 CH 2 OH<br />
100 o C<br />
This reaction would not be<br />
classified as either Zaitsev<br />
or Hofmann as the alkene<br />
generated is the only possible<br />
alkene.<br />
OH<br />
LiOCH 2 CH 3<br />
CH 3 CH 2 OH<br />
100 o C<br />
No reaction. There is no leaving group to<br />
undergo the elimination process. Hydroxide<br />
is not a suitable leaving group for the E2<br />
process. The alcohol would only deprotonate.<br />
NaOCH 3 in CH 3 OH<br />
OTs<br />
100 o C<br />
TsO = tosylate leaving group<br />
Zaitsev<br />
major<br />
minor<br />
Br<br />
KOC(CH 3 ) 3<br />
(CH 3 ) 3 COH<br />
100 o C<br />
major<br />
CH 2<br />
minor<br />
CH 3<br />
Hofmann<br />
9
13. Provide the major and minor alkenes from the following reactions and circle the major alkene. Indicate the level of<br />
substitution for this alkene. What is the dominant reaction mechanism for all of these reactions<br />
The most stable alkene is always the dominant product under conditions involving strong<br />
acid. The elimination of secondary and tertiary alcohols involves carbocations! The<br />
unwritten by-products for each reaction is water.<br />
H 3 PO 4<br />
heat<br />
No reaction. There is no leaving group. Cyclopentane will<br />
not react under these conditions.<br />
OH<br />
Et Et<br />
Et<br />
trisub.<br />
disub.<br />
CH 3<br />
OH<br />
H 3 PO 4<br />
heat<br />
+<br />
major<br />
minor<br />
OH<br />
H 2 SO 4<br />
heat<br />
major<br />
trans and cis-isomers<br />
+<br />
OH<br />
disub.<br />
H 3 PO 4<br />
heat<br />
H 2 SO 4<br />
heat<br />
trans and cis-isomers<br />
OH<br />
disub.<br />
H 2 SO 4<br />
heat<br />
OH<br />
H 3 PO 4<br />
heat<br />
There is no reaction in terms of forming a new alkene.<br />
There is no suitable β-hydrogen for straightforward<br />
removal.<br />
CH 3<br />
This is the exclusive alkene product<br />
for this reaction.<br />
tetrasub.<br />
major<br />
+<br />
minor<br />
monosub.<br />
monosub.<br />
CH2<br />
disub.<br />
minor<br />
10
14. Provide a mechanistic explanation for the following transformation. Identify nucleophiles and electrophiles. What is<br />
the name given to key process that leads into the alkene product<br />
electrophile<br />
H<br />
O<br />
H<br />
nucleophile<br />
CH 3<br />
CH 3<br />
CH 3<br />
CH 3<br />
H 2 SO 4<br />
heat<br />
H<br />
O<br />
H<br />
CH 3<br />
CH 3<br />
H<br />
O<br />
H<br />
E1 elimination pathway<br />
CH 3<br />
CH 3<br />
H<br />
H<br />
H<br />
CH 3<br />
CH 3<br />
H<br />
H<br />
H<br />
This hydrogen was always there. It did not appear<br />
from nowhere.<br />
The major transformation is a carbocation.<br />
11