Metabolism of purines and pyrimidines
Metabolism of purines and pyrimidines
Metabolism of purines and pyrimidines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
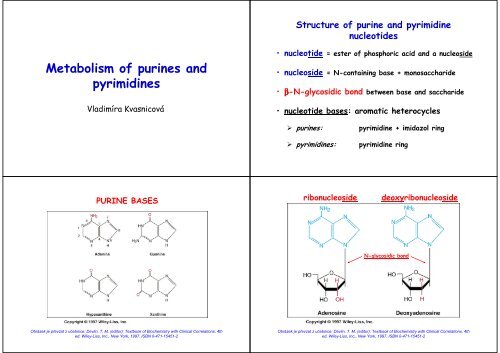
Structure <strong>of</strong> purine <strong>and</strong> pyrimidine<br />
nucleotides<br />
<strong>Metabolism</strong> <strong>of</strong> <strong>purines</strong> <strong>and</strong><br />
<strong>pyrimidines</strong><br />
Vladimíra Kvasnicová<br />
• nucleotide = ester <strong>of</strong> phosphoric acid <strong>and</strong> a nucleoside<br />
• nucleoside = N-containing base + monosaccharide<br />
• β-N-glycosidic bond between base <strong>and</strong> saccharide<br />
• nucleotide bases: aromatic heterocycles<br />
<strong>purines</strong>:<br />
<strong>pyrimidines</strong>:<br />
pyrimidine + imidazol ring<br />
pyrimidine ring<br />
PURINE BASES<br />
ribonucleoside<br />
deoxyribonucleoside<br />
N-glycosidic bond<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2
ibonucleotide<br />
deoxyribonucleotide<br />
PYRIMIDINE<br />
BASES<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
ribonucleosides<br />
deoxyribonucleoside<br />
Ribonucleotides<br />
* N-glycosidic bond<br />
* ester bond<br />
* anhydride bond<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor):<br />
Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th ed.<br />
Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2
Classification <strong>of</strong> nucleotides<br />
• purine nucleotides: contain adenine, guanine,<br />
hypoxanhine or xanthine<br />
• pyrimidine nucleosides: contain cytosine, uracil or<br />
thymine<br />
• ribonucleotides (saccharide = ribose)<br />
• deoxyribonukleotidy (saccharide = deoxyribose)<br />
formed by reduction <strong>of</strong> ribonucleoside<br />
diphosphates (NADPH)<br />
Purine nucleotides<br />
a) include an aromatic cycle in the structure<br />
b) can contain either adenine or thymine<br />
c) include N-glycosidic bond<br />
d) are composed <strong>of</strong> a nucleoside bound to<br />
phosphoric acid by an anhydride bond<br />
Purine nucleotides<br />
a) include an aromatic cycle in the structure<br />
b) can contain either adenine or thymine<br />
c) include N-glycosidic bond<br />
d) are composed <strong>of</strong> a nucleoside bound to<br />
phosphoric acid by an anhydride bond<br />
Pyrimidine nucleotides<br />
a) include an imidazol ring in the structure<br />
b) include thymidine- <strong>and</strong> cytidine monophosphate<br />
c) contain an ester bond<br />
d) can include 3 phosphate groups in their<br />
structure
Pyrimidine nucleotides<br />
a) include an imidazol ring in the structure<br />
b) include thymidine- <strong>and</strong> cytidine monophosphate<br />
c) contain an ester bond<br />
d) can include 3 phosphate groups in their<br />
structure<br />
Occurrence <strong>of</strong> nucleotides<br />
• essential for all cells<br />
• mainly 5´-nucleosidedi <strong>and</strong> triphosphates<br />
• ribonucleotides: concentration <strong>of</strong> a sum <strong>of</strong><br />
them is constant (mM), only their ratio varies<br />
(main ribonucleotide <strong>of</strong> cells: ATP)<br />
• deoxyribonucleotides: their concentration<br />
depends on a cell cycle (µM)<br />
Properties <strong>of</strong> nucleotides<br />
• strong absorption <strong>of</strong> UV radiation (260 nm)<br />
• <strong>purines</strong> are less stable under acidic conditions<br />
than <strong>pyrimidines</strong><br />
• polar terminal phosphate groups<br />
alternative names: adenylate or adenylic acid, ...<br />
Nucleotides in a metabolism<br />
1) energetic metabolism<br />
ATP = principal form <strong>of</strong> chemical energy<br />
available to cells – „as money <strong>of</strong> the cell“<br />
(30 kJ/mol / spliting <strong>of</strong>f phosphate)<br />
phosphotransferase reactions (kinases)<br />
muscle contraction, active transport<br />
2) monomeric units <strong>of</strong> RNA <strong>and</strong> DNA<br />
substrates: nucleoside triphosphates<br />
3) physiological mediators<br />
cAMP, cGMP („second messengers“)
4) components <strong>of</strong> coenzymes<br />
NAD, NADP, FAD, CoA<br />
3´-phosphoadenosine-5´-phosphosulfate (PAPS)<br />
used as the sulfate donor in metabolic reactions<br />
(sulfatation)<br />
5) activated intermediates<br />
UDP-Glc, GDP-Man, CMP-NANA<br />
CDP-choline, ethanolamine, diacylglycerol<br />
SAM → methylation<br />
PAPS → sulfatation<br />
6) allosteric efectors<br />
- regulation <strong>of</strong> key enzymes <strong>of</strong> metabolic<br />
pathways<br />
Obrázek je převzat z http://web.indstate.edu/thcme/mwking/amino-acid-metabolism.html (leden 2007)<br />
Purine <strong>and</strong> pyrimidine nucleotides can be<br />
used<br />
a) as nucleoside triphosphates for nucleic acid<br />
synthesis<br />
b) in energetic metabolism <strong>of</strong> cells<br />
c) for activation <strong>of</strong> metabolic intermediates <strong>of</strong><br />
saccharides <strong>and</strong> lipids<br />
d) in enzymatic reactions: some coenzymes are<br />
nucleotides<br />
Purine <strong>and</strong> pyrimidine nucleotides can be<br />
used<br />
a) as nucleoside triphosphates for nucleic acid<br />
synthesis<br />
b) in energetic metabolism <strong>of</strong> cells<br />
c) for activation <strong>of</strong> metabolic intermediates <strong>of</strong><br />
saccharides <strong>and</strong> lipids<br />
d) in enzymatic reactions: some coenzymes are<br />
nucleotides
PRPP = 5-phosphoribosyl-1-pyrophosphate<br />
• common substrate <strong>of</strong> both purine <strong>and</strong> pyrimidine<br />
synthesis<br />
• its synthesis is a key reaction <strong>of</strong> synthesis <strong>of</strong><br />
the nucleotides<br />
• function:<br />
regulation <strong>of</strong><br />
nucleotide synthesis<br />
substrate <strong>of</strong><br />
nucleotide synthesis<br />
• PRPP-synthetase is regulated by feed back<br />
inhibition by nucleoside di <strong>and</strong> triphosphates<br />
• precursors:<br />
* ribose-5-phosphate (from HMPP)<br />
* ribose-1-phosphate<br />
(phosphorolysis <strong>of</strong> nucleosides)<br />
PRPP = PRDP<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
Synthesis <strong>of</strong> purine nucleotides<br />
• de novo = new building <strong>of</strong> a nucleotide rings<br />
• salvage reactions=synthesis from bases or nucleosides<br />
less energy need than for de novo synthesis<br />
they inhibit de novo synthesis<br />
substrates: a) base (adenine, guanine, hypoxanthine)<br />
PRPP<br />
b) ribonucleosides<br />
ATP<br />
Synthesis <strong>of</strong> purine nucleotides de novo<br />
• high consumption <strong>of</strong> energy (ATP)<br />
• cytoplasm <strong>of</strong> many cells, mainly in the liver<br />
• substrates:<br />
• coenzymes:<br />
* 5-phosphoribosyl-1-diphosphate<br />
(= PRDP = PRPP)<br />
* amino acids<br />
(Gln, Gly, Asp)<br />
* tetrahydr<strong>of</strong>olate derivatives, CO 2<br />
* tetrahydr<strong>of</strong>olate (= THF)<br />
* NAD +
• important intermediates:<br />
5´-phosphoribosylamine<br />
inosine monophosphate (IMP)<br />
• products: nucleoside monophosphates (AMP, GMP)<br />
• interconversion <strong>of</strong> purine nucleotides:<br />
via IMP = common precursor <strong>of</strong> AMP <strong>and</strong> GMP<br />
(inosine monophosphate: base = hypoxanthine)<br />
C<br />
Y<br />
T<br />
O<br />
P<br />
L<br />
A<br />
S<br />
M<br />
Synthesis <strong>of</strong> purine nucleotides<br />
Obrázek převzat z http://web.indstate.edu/thcme/mwking/nucleotide-metabolism.html (leden 2007)<br />
IMP<br />
GMP<br />
AMP<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2
Synthesis <strong>of</strong> pyrimidine nucleotides<br />
• de novo = new building <strong>of</strong> a nucleotide rings<br />
• salvage reactions=synthesis from bases or nucleosides<br />
substrates:<br />
a) * base (not cytosine)<br />
* PRPP<br />
b) * ribonucleosides<br />
* ATP<br />
Synthesis <strong>of</strong> pyrimidine nucleotides de novo<br />
• cytoplasm <strong>of</strong> cells (exception: one enzyme is found at<br />
mitochondria /dihydroorotate-DH)<br />
• substrates:<br />
* carbamoyl phosphate (Gln,CO 2 ,2ATP)<br />
* aspartate<br />
* PRPP<br />
* methylene-THF (only for thimidine)<br />
Karbamoyl phosphate is formed in urea synthesis as well<br />
(only in mitochondria <strong>of</strong> hepatocytes)<br />
• important intermediates:<br />
• products:<br />
* orotic acid<br />
* orotidine monophosphate (OMP)<br />
* uridine monophosphate (UMP)<br />
* cytidine triphosphate (from UTP)<br />
* deoxythimidine monophosphate<br />
(from dUMP)<br />
C<br />
Y<br />
T<br />
O<br />
P<br />
L<br />
A<br />
S<br />
M<br />
Synthesis <strong>of</strong> pyrimidine nucleotides<br />
mitochondrion<br />
Obrázek převzat z http://web.indstate.edu/thcme/mwking/nucleotide-metabolism.html (leden 2007)
Synthesis <strong>of</strong> 2-deoxyribonucleotides<br />
enzyme: ribonucleotide reductase<br />
+ small protein „thioredoxin“<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2<br />
Synthesis <strong>of</strong> thymidine monophosphate<br />
Regulation <strong>of</strong> nucleotide synthesis<br />
• PRPP-synthetase is inhibited by both purine<br />
<strong>and</strong> pyrimidine nucleoside di- <strong>and</strong><br />
triphosphates<br />
• nucleotide synthesis: feed back inhibition<br />
• nucleoside diphosphate reductase:<br />
activated by nucleoside triphosphates,<br />
inhibited by deoxyadenosine triphosphate<br />
(dATP)<br />
Obrázek je převzat z učebnice: Devlin, T. M. (editor): Textbook <strong>of</strong> Biochemistry with Clinical Correlations, 4th<br />
ed. Wiley-Liss, Inc., New York, 1997. ISBN 0-471-15451-2
Regulation <strong>of</strong> nucleotide synthesis<br />
Degradation <strong>of</strong> <strong>purines</strong> <strong>and</strong> <strong>pyrimidines</strong><br />
regulatory enzyme<br />
activation<br />
inhibition<br />
• exogenous: mostly not used for resynthesis<br />
glutamine-PRPP<br />
amidotransferase<br />
(<strong>purines</strong>)<br />
carbamoylphosphate<br />
synthetase II<br />
= cytosolic<br />
(<strong>pyrimidines</strong>)<br />
• PRPP<br />
• PRPP<br />
• ATP<br />
• IMP, GMP,<br />
AMP<br />
(allosteric<br />
inhibition)<br />
• UTP<br />
• endogenous:<br />
enzymes<br />
* nucleases (split <strong>of</strong>f nucleic acids)<br />
* nucleotidases (...nucleotides)<br />
* nucleoside phosphorylases (nucleosides)<br />
* deaminase (adenosine)<br />
* xanthinoxidase<br />
(hypoxanthine, xanthine)<br />
inhibited by allopurinol (pharmacology)<br />
Degradation <strong>of</strong> <strong>purines</strong><br />
Degradation <strong>of</strong> <strong>pyrimidines</strong><br />
„uric acid“
• products:<br />
Principal differences between metabolism<br />
<strong>of</strong> <strong>purines</strong> <strong>and</strong> <strong>pyrimidines</strong><br />
<strong>purines</strong>→NH 3 , uric acid – it has antioxidative properties<br />
(partially excreted with urine; failure: hyperuricemia, gout)<br />
<strong>purines</strong><br />
<strong>pyrimidines</strong><br />
physiological range:<br />
serum 220 – 420 µmol/l (men)<br />
140 – 340 µmol/l (women)<br />
formation <strong>of</strong><br />
N-glycosidic<br />
bond<br />
in 1 st step <strong>of</strong> their<br />
biosynthesis<br />
(PRDP is the 1 st substrate)<br />
a heterocyclic ring is<br />
formed first, then it<br />
reacts with PRDP<br />
urine<br />
0,48 – 5,95 mmol/l<br />
location <strong>of</strong><br />
biosynthesis<br />
cytoplasm<br />
cytoplasm + 1 enzyme<br />
is in a mitochondrion<br />
<strong>pyrimidines</strong>: C, U → β-alanine, CO 2 , NH 3<br />
T → β-aminoisobutyrate, CO 2 , NH 3<br />
products <strong>of</strong><br />
degradation<br />
uric acid<br />
(poor solubility in H 2 O),<br />
NH 3<br />
CO 2 , NH 3 , β-AMK<br />
(soluble in H 2 O)<br />
Synthesis <strong>of</strong> nucleotides<br />
Synthesis <strong>of</strong> nucleotides<br />
a) uses products <strong>of</strong> pentose cycle<br />
b) includes phosphoribosyl diphosphate (PRDP =<br />
PRPP) as a substrate<br />
c) needs derivatives <strong>of</strong> folic acid<br />
d) proceeds in a cytoplasm only<br />
a) uses products <strong>of</strong> pentose cycle<br />
b) includes phosphoribosyl diphosphate (PRDP =<br />
PRPP) as a substrate<br />
c) needs derivatives <strong>of</strong> folic acid<br />
d) proceeds in a cytoplasm only
Synthesis <strong>of</strong> purine nucleotides<br />
Synthesis <strong>of</strong> purine nucleotides<br />
a) uses ammonia as a nitrogen donor<br />
b) proceeds in a cytoplasm<br />
c) can start from nucleosides produced by<br />
degradation <strong>of</strong> nucleic acids<br />
d) includes uric acid as an intermediate<br />
a) uses ammonia as a nitrogen donor<br />
b) proceeds in a cytoplasm<br />
c) can start from nucleosides produced by<br />
degradation <strong>of</strong> nucleic acids<br />
d) includes uric acid as an intermediate<br />
Synthesis <strong>of</strong> pyrimidine nucleotides<br />
Synthesis <strong>of</strong> pyrimidine nucleotides<br />
a) starts by the reaction: PRDP + glutamine<br />
b) proceeds only in a cytoplasm <strong>of</strong> cells<br />
c) includes orotic acid as an intermediate<br />
d) includes inosine monophosphate as an<br />
intermediate<br />
a) starts by the reaction: PRDP + glutamine<br />
b) proceeds only in a cytoplasm <strong>of</strong> cells<br />
c) includes orotic acid as an intermediate<br />
d) includes inosine monophosphate as an<br />
intermediate
In a degradation <strong>of</strong> purine nucleotides<br />
In a degradation <strong>of</strong> purine nucleotides<br />
a) ammonia is released<br />
b) CO 2 is produced<br />
c) the enzyme xanthine oxidase participates<br />
d) uric acid is produced as the end product<br />
a) ammonia is released<br />
b) CO 2 is produced<br />
c) the enzyme xanthine oxidase participates<br />
d) uric acid is produced as the end product<br />
In a degradation <strong>of</strong> pyrimidine nucleotides<br />
In a degradation <strong>of</strong> pyrimidine nucleotides<br />
a) β-amino acids are produced<br />
b) the enzyme xanthine oxidase participates<br />
c) orotic acid is formed<br />
d) ammonia is produced<br />
a) β-amino acids are produced<br />
b) the enzyme xanthine oxidase participates<br />
c) orotic acid is formed<br />
d) ammonia is produced