Molecular Dynamics Chapter 2. Molecular Dynamics in Various ...
Molecular Dynamics Chapter 2. Molecular Dynamics in Various ...
Molecular Dynamics Chapter 2. Molecular Dynamics in Various ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Molecular</strong> <strong>Dynamics</strong><br />
<strong>Chapter</strong> <strong>2.</strong> <strong>Molecular</strong> <strong>Dynamics</strong> <strong>in</strong> <strong>Various</strong> Ensembles<br />
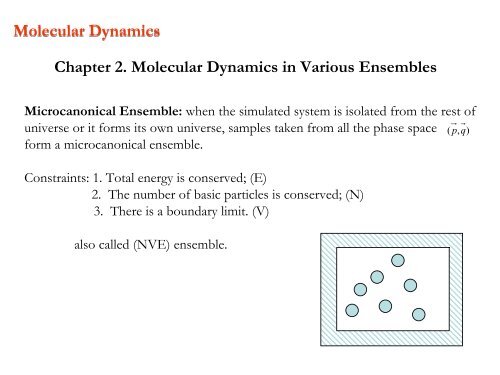
Microcanonical Ensemble: when the simulated system is isolated from the rest of<br />
universe or it forms its own universe, samples taken from all the phase space ( p,<br />
q)<br />
form a microcanonical ensemble.<br />
Constra<strong>in</strong>ts: 1. Total energy is conserved; (E)<br />
<strong>2.</strong> The number of basic particles is conserved; (N)<br />
3. There is a boundary limit. (V)<br />
also called (NVE) ensemble.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Basic Thermodynamics<br />
Equilibrium: when samples can be mean<strong>in</strong>gfully used for state-function<br />
(thermodynamic property) measurements, system is <strong>in</strong> equilibrium.<br />
1. It is a relative concept, dependent on overall sampl<strong>in</strong>g length.<br />
<strong>2.</strong> It is a concept conditioned by ergodic requirements.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Basic Thermodynamics<br />
Second law of thermodynamics: maximum entropy theorem<br />
Dur<strong>in</strong>g the course of system’s approach<strong>in</strong>g the equilibrium state, entropy is<br />
maximized when the total energy is conserved. (NVE)<br />
Alternative: m<strong>in</strong>imum energy theorem<br />
Dur<strong>in</strong>g the course of system’s approach<strong>in</strong>g the equilibrium state,<br />
energy is m<strong>in</strong>imized when the entropy is conserved (NVS).<br />
Entropy: a measure of system diversity (disorder).<br />
dQ<br />
dS = ( )<br />
T<br />
resersible<br />
dE<br />
dV<br />
= dQ + dW<br />
= 0 , dW = 0<br />
1 / = (<br />
dS<br />
dE<br />
)<br />
T<br />
N , V
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Basic Thermodynamics – Statistical Mechanics<br />
1 / =<br />
(<br />
dS<br />
dE<br />
)<br />
T<br />
N , V<br />
NkT<br />
= ∑<br />
2<br />
m i<br />
v i<br />
For a large subset of a huge system, <strong>in</strong> the equilibrium state,<br />
Total E= E 1 +E 2 under mean field condition<br />
If Ω( E,<br />
V , N ) represents the occurrence probability (or degeneracy)<br />
∑<br />
Γ<br />
Ω ( E,<br />
V , N)<br />
= δ ( H ( Γ)<br />
− E)<br />
s = k lnΩ
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Basic Thermodynamics – Statistical Mechanics<br />
1 / =<br />
(<br />
dS<br />
dE<br />
)<br />
T<br />
N , V<br />
NkT<br />
= ∑<br />
2<br />
m i<br />
v i<br />
For a large subset of a huge system, <strong>in</strong> the equilibrium state,<br />
Total E= E 1 +E 2 under mean field condition<br />
s<br />
= k ln<br />
Ω<br />
⎛ ∂k<br />
ln Ω(<br />
E , E − E<br />
⎜<br />
⎝ ∂E1<br />
) ⎞<br />
⎟<br />
⎠<br />
1 1<br />
=<br />
N , V , E<br />
0<br />
⎛ ∂k<br />
ln Ω(<br />
E1 , V , N)<br />
⎜<br />
⎝ ∂E1<br />
⎞<br />
⎟<br />
⎠<br />
N , V , E<br />
= β<br />
β = 1/(<br />
kT )
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Basic Statistical Mechanics<br />
Simple example: (NVE)<br />
Two state potential energy function: E = (E 0 , E 1 ) for two recognizable particles<br />
Total energy E 0 + E 1<br />
E 1<br />
E 1<br />
Ω = 2<br />
E 0<br />
E 0<br />
s<br />
=<br />
k<br />
ln 2<br />
k<br />
= 1.38E<br />
−<br />
23
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Basic Statistical Mechanics<br />
Simple example: (NVE)<br />
Two state potential energy function: E = (0, E 0 , E 1 , E 0 + E 1 ) for two recognizable<br />
particles. Total energy E 0 + E 1<br />
E 0 + E 1<br />
E 1<br />
4<br />
E 0<br />
0<br />
Ω =<br />
s<br />
=<br />
k<br />
ln 4<br />
k<br />
= 1.38E<br />
−<br />
23
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Microcanonical Ensemble<br />
Microcanonical Ensemble <strong>in</strong> MD simulation:<br />
1. The occurrence probability is <strong>in</strong>dependent of subset of energies.<br />
<strong>2.</strong> Ensemble property is dependent on the maximum entropy.<br />
3. Easy to implement.<br />
4. Difficult to control macroscopic condition.<br />
5. It can be used as thermo reservoir for canonical ensemble simulations.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>2<br />
Canonical Ensemble<br />
Canonical Ensemble: when the simulated system is embedded <strong>in</strong> an <strong>in</strong>f<strong>in</strong>ite<br />
heat bath, but does not have particle exchange with this bath, it forms a<br />
canonical ensemble.<br />
Constra<strong>in</strong>ts: 1. System temperature is conserved (not absolutely constant); (T)<br />
<strong>2.</strong> The number of basic particles is conserved; (N)<br />
3. There is a boundary limit (V); or there is a constant pressure (P)<br />
also called (NVT) ensemble or (NPT) ensemble.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Canonical Ensemble<br />
Canonical ensemble can be considered as a sub-microcanonical ensemble, where<br />
particle collisions occur on the “boundary”.<br />
In practice, the temperature buffer region can be flexibly designed and its<br />
boundary with the simulated region can also be flexibly designed.<br />
Centers of canonical ensemble algorithm developments
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Canonical Ensemble<br />
Canonical ensemble can be derived from microcanonical ensemble, too.<br />
EB<br />
E<br />
A<br />
E = E A<br />
+ EB<br />
For a given system energy E A , reservoir probability determ<strong>in</strong>es its probability.<br />
Ω(<br />
E − E ) A<br />
In micro-canonical ensemble, higher energy has larger probability. Why <br />
So lower system energy should have larger probability. Why
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Canonical Ensemble<br />
EB<br />
E<br />
A<br />
E = E A<br />
+ EB<br />
For a given system energy E A , reservoir probability determ<strong>in</strong>es its probability.<br />
Because simulated system is much smaller than the buffer portion,<br />
Taylor expansion:<br />
∂ ln Ω(<br />
E)<br />
ln Ω(<br />
E − E<br />
A<br />
) = ln Ω(<br />
E)<br />
− E<br />
A<br />
= ln Ω(<br />
E)<br />
− E<br />
A<br />
/ kT<br />
∂E<br />
Probability for the system with energy E A :<br />
P(<br />
E<br />
A<br />
)<br />
=<br />
exp( −E<br />
∑<br />
i<br />
A<br />
exp( −E<br />
/<br />
i<br />
kT )<br />
/ kT )
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Canonical Ensemble<br />
P(<br />
E<br />
A<br />
)<br />
=<br />
exp( −E<br />
∑<br />
i<br />
A<br />
exp( −E<br />
/<br />
i<br />
kT )<br />
/ kT )<br />
We should learn from this derivation on canonical ensemble algorithm design:<br />
1. The thermal reservoir is essential, because it governs the Boltzmann<br />
distribution.<br />
<strong>2.</strong> The thermal volume of the thermal reservoir should be large enough to<br />
reflect the Taylor expension.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Temperature <strong>in</strong> Canonical Ensemble<br />
3<br />
E K<br />
=<br />
2<br />
NkT<br />
Canonical ensemble is not absolutely constant-temperature or<br />
isok<strong>in</strong>etic ensemble.<br />
Maxwell-Boltzmann distribution:<br />
ρ(|<br />
p<br />
|)<br />
=<br />
⎛<br />
⎜<br />
⎝<br />
β<br />
2πm<br />
⎞<br />
⎟<br />
⎠<br />
3 / 2<br />
exp<br />
[ | | /(2 )]<br />
2<br />
− β p m
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Thermal Reservoir Algorithm Design<br />
• Reservoir can be flexible, represented by other natures of particle, force,<br />
or <strong>in</strong>teractions.<br />
<strong>2.</strong> Coupl<strong>in</strong>g boundary can also be flexible, not restra<strong>in</strong>ed by the spatial boundary.<br />
3. Computation overhead should be small.<br />
4. Preserve canonical ensemble: temperature<br />
and Maxwell-Boltzmann distribution.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
The Simplest Algorithm: Velocity Re-scal<strong>in</strong>g<br />
ΔT<br />
=<br />
N<br />
1 2 i<br />
λ<br />
i<br />
2<br />
∑<br />
i=<br />
1<br />
3<br />
m<br />
(<br />
Nk<br />
v<br />
B<br />
)<br />
2<br />
−<br />
1<br />
2<br />
N<br />
∑<br />
i=<br />
1<br />
2<br />
3<br />
m v<br />
i<br />
Nk<br />
2<br />
i<br />
B<br />
ΔT<br />
=<br />
( λ<br />
2 −1)<br />
T ( t)<br />
λ =<br />
T new<br />
/ T ( t)<br />
T<br />
=<br />
new<br />
T t arg et
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
The Simplest Algorithm: Velocity Re-scal<strong>in</strong>g<br />
1. Not real canonical ensemble, although it is close.<br />
<strong>2.</strong> Maxwell-Boltzmann distribution can not be guaranteed. Scal<strong>in</strong>g frequency is not<br />
robust (ad hoc).<br />
3. Propagation stability can be destroyed.<br />
4. How to make judgments dur<strong>in</strong>g the simulations <br />
k<strong>in</strong>etic energy and potential energy exchange.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Berendsen Thermostat<br />
Velocity Re-scal<strong>in</strong>g scheme has absolute target temperature every time-step.<br />
To generate a temperature fluctuation, the difference between the target temperature<br />
and the <strong>in</strong>stantaneous temperature can be used to drive the temperature change<br />
dT ( t)<br />
dt<br />
= 1<br />
( T<br />
τ<br />
bath −<br />
T ( t))<br />
The temperature change between successive time steps is:<br />
ΔT<br />
=<br />
δt<br />
( Tbath − T ( t))<br />
τ
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Berendsen Thermostat<br />
In practice, we need to perform a temperature dependent velocity rescal<strong>in</strong>g:<br />
2<br />
λ<br />
δt<br />
⎛ Tbath<br />
⎞<br />
= 1+<br />
⎜ −1⎟<br />
τ ⎝ T ( t)<br />
⎠<br />
When the coupl<strong>in</strong>g parameter is equal to the time step, berendsen thermostat is<br />
equivalent to the velocity rescal<strong>in</strong>g method.<br />
For large uniform systems, scal<strong>in</strong>g ratio δt/τ = 0.0025 can produce a pseudo<br />
Maxwell-Boltzmann Distribution.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Local Temperature Trapp<strong>in</strong>g<br />
The temperature fluctuation is important for the<br />
trapped k<strong>in</strong>etic energy to be released.<br />
When a large scal<strong>in</strong>g ratio is applied, the<br />
temperature fluctuation will be prohibited.<br />
When a small scal<strong>in</strong>g ratio is applied, the<br />
temperature averag<strong>in</strong>g can not be easily<br />
guaranteed.<br />
Hot spot<br />
Intr<strong>in</strong>sic problem: thermo-diffusivity is low.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Stochastic Collision method:<br />
Anderson Thermostat<br />
The velocities of randomly selected particles are randomly reset, based on<br />
Maxwell-Boltzmann distribution.<br />
Random selection: if random number [0, 1] > νdt,<br />
ν is stochastic collision frequency<br />
dt is the time step.<br />
the correspond<strong>in</strong>g particle is selected.<br />
Random Reset:<br />
distribution.<br />
Gaussian(velocity) based on Maxwell-Boltzmann
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Integration: Velocity Verlet Algorithm<br />
r<br />
v<br />
F<br />
t-Δt t t+Δt<br />
Given current position,<br />
velocity, and force
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Integration: Velocity Verlet Algorithm<br />
r<br />
v<br />
F<br />
t-Δt t t+Δt<br />
Compute new position
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Integration: Velocity Verlet Algorithm<br />
r<br />
v<br />
F<br />
t-Δt t t+Δt<br />
Compute velocity at half step
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Integration: Velocity Verlet Algorithm<br />
r<br />
v<br />
F<br />
t-Δt t t+Δt<br />
Compute force at new position
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Integration: Velocity Verlet Algorithm<br />
r<br />
v<br />
F<br />
t-Δt t t+Δt<br />
Compute velocity at full step<br />
At this step, some particles’ velocities will be reset based on<br />
the Anderson formulation.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Integration: Velocity Verlet Algorithm<br />
r<br />
v<br />
F<br />
t-2Δt t-Δt t t+Δt<br />
Advance to next time step,<br />
repeat
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Stochastic Collision method:<br />
Anderson Thermostat<br />
The Anderson process is equivalent to the Monte Carlo move.<br />
This process becomes a Markov process (no memory). And the<br />
canonical distribution (Boltzmann distribution) can be guaranteed.<br />
Question: Can we provide random <strong>in</strong>put from Force rather than<br />
Velocity If so, which <strong>in</strong>tegrator is ideal What is the advantage and<br />
What is the disadvantage
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Stochastic Collision method:<br />
Anderson Thermostat<br />
Frequency <strong>in</strong>fluence on the phase space exploration:<br />
If frequency is too high, collision will dom<strong>in</strong>ate and <strong>in</strong>hibit an efficient<br />
exploration of the phase space to meet the ergodic requirement.<br />
If frequency is too low, the canonical distribution will be formed slowly<br />
to meet the canonical requirement.<br />
Suggested collision rate:<br />
ρ<br />
Cλ<br />
T<br />
1/ 3 2 / 3<br />
N
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Extended Hamiltonian Approach<br />
H<br />
1 2<br />
d s r<br />
H M k s s<br />
2<br />
extended<br />
=<br />
o<br />
+<br />
r<br />
+<br />
r r − +<br />
2<br />
dt<br />
1<br />
2<br />
U<br />
bias<br />
( s,<br />
t)<br />
Any targeted property: temperature, pressure, energy difference,<br />
geometry, … can be coupled to the orig<strong>in</strong>al Hamiltonian to achieve<br />
the desired distributions.<br />
It is equivalent to the biased Monte Carlo.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> 1.1<br />
Nose-Hoover Thermostat<br />
H<br />
extended<br />
N<br />
= U<br />
o<br />
+ ∑<br />
i=<br />
1<br />
p<br />
2<br />
i<br />
2m s<br />
i<br />
2<br />
r<br />
+<br />
1<br />
2<br />
M<br />
r<br />
ds<br />
dt<br />
r<br />
2<br />
+<br />
L<br />
ln sr<br />
β<br />
1. S r has to be greater than 1. (Lagrangian multiplier)<br />
<strong>2.</strong> S r plays a role <strong>in</strong> rescal<strong>in</strong>g the time-step.<br />
3. So, the real time step fluctuates.<br />
4. The Q is equivalent to the Q of the canonical ensemble.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>2<br />
Grand Canonical Ensemble<br />
Grand Canonical Ensemble: when the simulated system is embedded <strong>in</strong> an<br />
<strong>in</strong>f<strong>in</strong>ite heat bath, and can exchange particle with the <strong>in</strong>f<strong>in</strong>ite particle reservoir, it<br />
forms a grand canonical ensemble.<br />
Constra<strong>in</strong>ts: 1. System temperature is conserved (not absolutely constant); (T)<br />
<strong>2.</strong> The chemical potential of the particle reservoir is constant (μ)<br />
3. There is a boundary limit (V); or there is a constant pressure (P)<br />
also called (GVT) ensemble or (GPT) ensemble.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Grand Canonical Ensemble<br />
Canonical ensemble can be considered as a sub-microcanonical ensemble, where<br />
particle collisions and exchanges occur on the “boundary”.<br />
In practice, the reservoir buffer region can be flexibly designed and its<br />
boundary with the simulated region can also be flexibly designed.<br />
Centers of grand canonical ensemble algorithm developments
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Canonical Ensemble<br />
P(<br />
E<br />
A<br />
)<br />
=<br />
exp( −E<br />
∑<br />
i<br />
A<br />
exp( −E<br />
/<br />
i<br />
kT )<br />
/ kT )<br />
How to write Grand Canonical Ensemble partition function:<br />
P<br />
∞<br />
∑<br />
N = 0<br />
=<br />
( N!)<br />
−1<br />
V<br />
N<br />
z<br />
Q<br />
N<br />
∫<br />
μVT<br />
dr<br />
exp( −βE)<br />
z<br />
=<br />
( h<br />
2<br />
exp( βμ )<br />
/ 2πmk<br />
T )<br />
B<br />
3/ 2
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
How to Realize Grand Canonical Ensemble <strong>in</strong> Monte Carlo <br />
1. Internal Displacements<br />
ρ<br />
accp<br />
=<br />
m<strong>in</strong>( 1,exp( −βE))<br />
<strong>2.</strong> Insertion and removal<br />
ρ<br />
ρ<br />
accp<br />
accp<br />
=<br />
−<br />
m<strong>in</strong>( 1,exp( −β<br />
( En<br />
1<br />
+ u − En))<br />
=<br />
+<br />
m<strong>in</strong>( 1,exp( −β<br />
( En<br />
1<br />
− u − En))
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Hybrid Monte Carlo for Grand Canonical Ensemble <br />
1. Internal Displacements Canonical ensemble <strong>Molecular</strong> <strong>Dynamics</strong><br />
<strong>2.</strong> Insertion and removal ρaccp<br />
= m<strong>in</strong>( 1,exp( −β<br />
( En−<br />
1<br />
+ u − En))<br />
ρ<br />
accp<br />
=<br />
+<br />
m<strong>in</strong>( 1,exp( −β<br />
( En<br />
1<br />
− u − En))
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Extended Hamiltonian to Realize Grand Canonical Ensemble
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Extended Hamiltonian to Realize Grand Canonical Ensemble<br />
One particle scheme:<br />
H<br />
extended<br />
1 dsr<br />
= H<br />
o<br />
( n −1)<br />
+ M<br />
r<br />
+ sr<br />
( U<br />
o<br />
− μ ) + μ<br />
2 dt<br />
0≤ S r<br />
≤1<br />
2
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Extended Hamiltonian to Realize Grand Canonical Ensemble<br />
One particle scheme:<br />
H<br />
extended<br />
1 dsr<br />
= H<br />
o<br />
( n −1)<br />
+ M<br />
r<br />
+ sr<br />
( U<br />
o<br />
− μ ) + μ<br />
2 dt<br />
0≤ S r<br />
≤1<br />
2<br />
Drawbacks: 1. Particle number fluctuation magnitude can be larger<br />
than 1.<br />
<strong>2.</strong> When Sr = 0, it can take long simulation time for the ghost<br />
particle to locate a position aga<strong>in</strong>. Why <br />
Interaction S<strong>in</strong>gularity.
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Extended Hamiltonian to Realize Grand Canonical Ensemble<br />
One particle scheme:<br />
H<br />
extended<br />
1 dsr<br />
= H<br />
o<br />
( n −1)<br />
+ M<br />
r<br />
+ sr<br />
( U<br />
o<br />
− μ ) + μ<br />
2 dt<br />
2<br />
0≤ S r<br />
≤1<br />
How to solve the <strong>in</strong>teraction s<strong>in</strong>gularity, when S r<br />
= 0 <br />
Two General Strategies: 1. Make the switch<strong>in</strong>g S r<br />
(U o<br />
-μ) nonl<strong>in</strong>ear.<br />
<strong>2.</strong> Adiabatic switch<strong>in</strong>g method
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Extended Hamiltonian to Realize Grand Canonical Ensemble<br />
One particle scheme:<br />
H<br />
extended<br />
1 dsr<br />
= H<br />
o<br />
( n −1)<br />
+ M<br />
r<br />
+ srU<br />
o<br />
− srμ + μ<br />
2 dt<br />
2<br />
0≤ S r<br />
≤1<br />
Nonl<strong>in</strong>ear switch<strong>in</strong>g S r<br />
U o<br />
Orig<strong>in</strong>al Lennard-Jones <strong>in</strong>teraction:<br />
Nonl<strong>in</strong>ear Switch<strong>in</strong>g (Softcore potential):<br />
⎡ A B<br />
ε ⎢ 12<br />
− 6<br />
⎣r<br />
r<br />
⎤<br />
⎥<br />
⎦<br />
s<br />
r<br />
⎧ ⎡<br />
⎨ε<br />
⎢<br />
⎩ ⎣<br />
A<br />
+ σ (1 −<br />
−<br />
B<br />
+ σ (1 −<br />
2<br />
6 2<br />
( r sr<br />
)) ( r sr<br />
))<br />
3<br />
⎤⎫<br />
⎥⎬<br />
⎦⎭
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Extended Hamiltonian to Realize Grand Canonical Ensemble<br />
⎧ ⎡<br />
⎨ε<br />
⎢<br />
⎩ ⎣<br />
A<br />
B<br />
−<br />
2<br />
6 2<br />
( r + σ (1 − sr<br />
)) ( r + σ (1 − sr<br />
))<br />
3<br />
⎤⎫<br />
⎥⎬<br />
⎦⎭<br />
Another Advantage:
<strong>Molecular</strong> <strong>Dynamics</strong> <strong>Chapter</strong> <strong>2.</strong>1<br />
Extended Hamiltonian to Realize Grand Canonical Ensemble<br />
Adiabatic switch<strong>in</strong>g method: Extended Hamiltonian Hybrid Monte Carlo<br />
H<br />
extended<br />
1 dsr<br />
= H<br />
o<br />
( n −1)<br />
+ M<br />
r<br />
+ sr<br />
( U<br />
o<br />
− μ ) + μ<br />
2 dt<br />
2<br />
1. When Sr = 0 or 1, at a pre-set time <strong>in</strong>terval N*τ, fix<strong>in</strong>g the position<br />
of the ghost particle.<br />
<strong>2.</strong> Adiabatically switch Sr from 0 to 1 or switch Sr from 1 to 0, the<br />
energy change will be E − or E −<br />
n−1<br />
E n<br />
n+1<br />
E n<br />
ρ<br />
ρ<br />
accp<br />
accp<br />
=<br />
−<br />
m<strong>in</strong>( 1,exp( −β<br />
( En<br />
1<br />
+ u − En))<br />
=<br />
+<br />
m<strong>in</strong>( 1,exp( −β<br />
( En<br />
1<br />
− u − En))