Untitled - Laboratory of Neurophysics and Physiology
Untitled - Laboratory of Neurophysics and Physiology
Untitled - Laboratory of Neurophysics and Physiology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
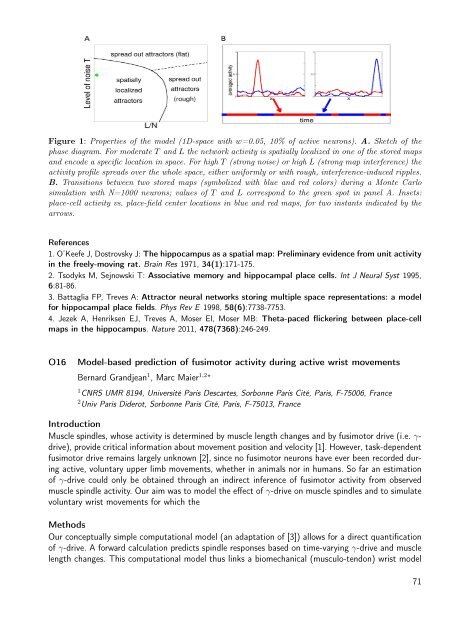
Figure 1: Properties <strong>of</strong> the model (1D-space with w=0.05, 10% <strong>of</strong> active neurons). A. Sketch <strong>of</strong> the<br />
phase diagram. For moderate T <strong>and</strong> L the network activity is spatially localized in one <strong>of</strong> the stored maps<br />
<strong>and</strong> encode a specific location in space. For high T (strong noise) or high L (strong map interference) the<br />
activity pr<strong>of</strong>ile spreads over the whole space, either uniformly or with rough, interference-induced ripples.<br />
B. Transitions between two stored maps (symbolized with blue <strong>and</strong> red colors) during a Monte Carlo<br />
simulation with N=1000 neurons; values <strong>of</strong> T <strong>and</strong> L correspond to the green spot in panel A. Insets:<br />
place-cell activity vs. place-field center locations in blue <strong>and</strong> red maps, for two instants indicated by the<br />
arrows.<br />
References<br />
1. O’Keefe J, Dostrovsky J: The hippocampus as a spatial map: Preliminary evidence from unit activity<br />
in the freely-moving rat. Brain Res 1971, 34(1):171-175.<br />
2. Tsodyks M, Sejnowski T: Associative memory <strong>and</strong> hippocampal place cells. Int J Neural Syst 1995,<br />
6:81-86.<br />
3. Battaglia FP, Treves A: Attractor neural networks storing multiple space representations: a model<br />
for hippocampal place fields. Phys Rev E 1998, 58(6):7738-7753.<br />
4. Jezek A, Henriksen EJ, Treves A, Moser EI, Moser MB: Theta-paced flickering between place-cell<br />
maps in the hippocampus. Nature 2011, 478(7368):246-249.<br />
O16<br />
Model-based prediction <strong>of</strong> fusimotor activity during active wrist movements<br />
Bernard Gr<strong>and</strong>jean 1 , Marc Maier 1,2⋆<br />
1 CNRS UMR 8194, Université Paris Descartes, Sorbonne Paris Cité, Paris, F-75006, France<br />
2 Univ Paris Diderot, Sorbonne Paris Cité, Paris, F-75013, France<br />
Introduction<br />
Muscle spindles, whose activity is determined by muscle length changes <strong>and</strong> by fusimotor drive (i.e. γ-<br />
drive), provide critical information about movement position <strong>and</strong> velocity [1]. However, task-dependent<br />
fusimotor drive remains largely unknown [2], since no fusimotor neurons have ever been recorded during<br />
active, voluntary upper limb movements, whether in animals nor in humans. So far an estimation<br />
<strong>of</strong> γ-drive could only be obtained through an indirect inference <strong>of</strong> fusimotor activity from observed<br />
muscle spindle activity. Our aim was to model the effect <strong>of</strong> γ-drive on muscle spindles <strong>and</strong> to simulate<br />
voluntary wrist movements for which the<br />
Methods<br />
Our conceptually simple computational model (an adaptation <strong>of</strong> [3]) allows for a direct quantification<br />
<strong>of</strong> γ-drive. A forward calculation predicts spindle responses based on time-varying γ-drive <strong>and</strong> muscle<br />
length changes. This computational model thus links a biomechanical (musculo-tendon) wrist model<br />
71