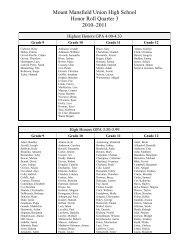
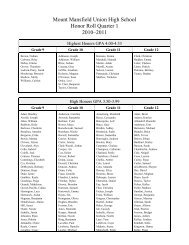
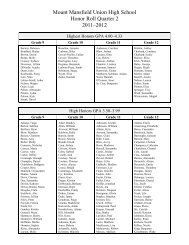
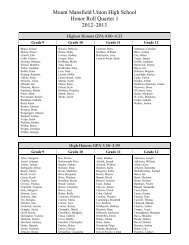
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4. Balance <strong>and</strong> type the following reaction, <strong>and</strong> then draw a fully labeled energy level diagram. Include bond<br />
making <strong>and</strong> bond breaking, activation energy, total energy, starting <strong>and</strong> ending materials. Endo- or exothermic?<br />
C2H5OH + O2 � CO2 + H2O<br />
5. Here is a partial list of elements in order of decreasing "activity". Determine if a reaction will occur. If so,<br />
write the completed, balanced equation.<br />
Metals: Li K Ca Na Mg Al Zn Cr Fe Ni Sn Pb H Cu Ag Hg Pt Au<br />
Non-metals: F Cl Br I<br />
a. Mg + AlCl3 �<br />
b. FeSO4 + Ni �<br />
c. NaI + Cl2 �<br />
d. HCl + Al �<br />
10