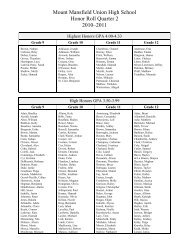
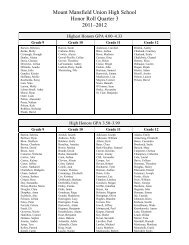
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
e. H2(g) + O2(g) ----><br />
f. Zn(s) + AgNO3(aq) ----><br />
g. C5H10(s) + O2(g) ----><br />
h. LiOH(aq) + H2SO4(aq) ----><br />
i. NH4Br(s) + NaOH(aq) ----><br />
j. MgCO3(s) + H2SO4(aq) ----><br />
4. Write the two half-cell reactions that take place when zinc metal is placed into a solution of silver nitrate, <strong>and</strong><br />
label them appropriately as "reduction" or "oxidation".<br />
8