post-splenectomy vaccine prophylaxis - SurgicalCriticalCare.net
post-splenectomy vaccine prophylaxis - SurgicalCriticalCare.net
post-splenectomy vaccine prophylaxis - SurgicalCriticalCare.net
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They<br />
are intended to serve as a general statement regarding appropriate patient care practices based upon the available medical<br />
literature and clinical expertise at the time of development. They should not be considered to be accepted protocol or policy, nor are<br />
intended to replace clinical judgment or dictate care of individual patients.<br />
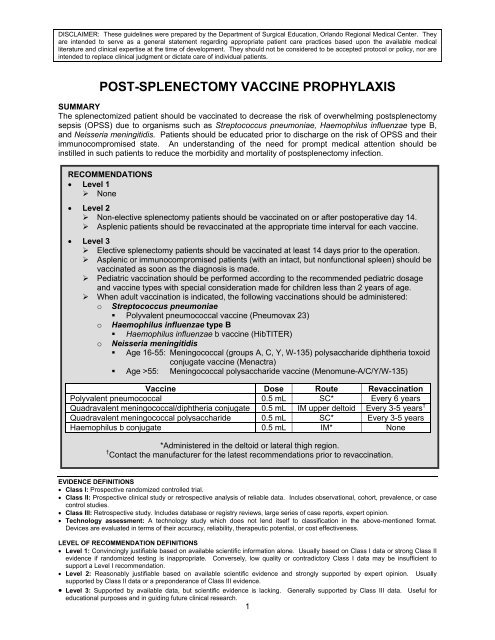
POST-SPLENECTOMY VACCINE PROPHYLAXIS<br />
SUMMARY<br />
The splenectomized patient should be vaccinated to decrease the risk of overwhelming <strong>post</strong><strong>splenectomy</strong><br />
sepsis (OPSS) due to organisms such as Streptococcus pneumoniae, Haemophilus influenzae type B,<br />
and Neisseria meningitidis. Patients should be educated prior to discharge on the risk of OPSS and their<br />
immunocompromised state. An understanding of the need for prompt medical attention should be<br />
instilled in such patients to reduce the morbidity and mortality of <strong>post</strong><strong>splenectomy</strong> infection.<br />
RECOMMENDATIONS<br />
• Level 1<br />
‣ None<br />
• Level 2<br />
‣ Non-elective <strong>splenectomy</strong> patients should be vaccinated on or after <strong>post</strong>operative day 14.<br />
‣ Asplenic patients should be revaccinated at the appropriate time interval for each <strong>vaccine</strong>.<br />
• Level 3<br />
‣ Elective <strong>splenectomy</strong> patients should be vaccinated at least 14 days prior to the operation.<br />
‣ Asplenic or immunocompromised patients (with an intact, but nonfunctional spleen) should be<br />
vaccinated as soon as the diagnosis is made.<br />
‣ Pediatric vaccination should be performed according to the recommended pediatric dosage<br />
and <strong>vaccine</strong> types with special consideration made for children less than 2 years of age.<br />
‣ When adult vaccination is indicated, the following vaccinations should be administered:<br />
o Streptococcus pneumoniae<br />
• Polyvalent pneumococcal <strong>vaccine</strong> (Pneumovax 23)<br />
o Haemophilus influenzae type B<br />
• Haemophilus influenzae b <strong>vaccine</strong> (HibTITER)<br />
o Neisseria meningitidis<br />
• Age 16-55: Meningococcal (groups A, C, Y, W-135) polysaccharide diphtheria toxoid<br />
conjugate <strong>vaccine</strong> (Menactra)<br />
• Age >55: Meningococcal polysaccharide <strong>vaccine</strong> (Menomune-A/C/Y/W-135)<br />
Vaccine Dose Route Revaccination<br />
Polyvalent pneumococcal 0.5 mL SC* Every 6 years<br />
Quadravalent meningococcal/diphtheria conjugate 0.5 mL IM upper deltoid Every 3-5 years †<br />
Quadravalent meningococcal polysaccharide 0.5 mL SC* Every 3-5 years<br />
Haemophilus b conjugate 0.5 mL IM* None<br />
*Administered in the deltoid or lateral thigh region.<br />
† Contact the manufacturer for the latest recommendations prior to revaccination.<br />
EVIDENCE DEFINITIONS<br />
• Class I: Prospective randomized controlled trial.<br />
• Class II: Prospective clinical study or retrospective analysis of reliable data. Includes observational, cohort, prevalence, or case<br />
control studies.<br />
• Class III: Retrospective study. Includes database or registry reviews, large series of case reports, expert opinion.<br />
• Technology assessment: A technology study which does not lend itself to classification in the above-mentioned format.<br />
Devices are evaluated in terms of their accuracy, reliability, therapeutic potential, or cost effectiveness.<br />
LEVEL OF RECOMMENDATION DEFINITIONS<br />
• Level 1: Convincingly justifiable based on available scientific information alone. Usually based on Class I data or strong Class II<br />
evidence if randomized testing is inappropriate. Conversely, low quality or contradictory Class I data may be insufficient to<br />
support a Level I recommendation.<br />
• Level 2: Reasonably justifiable based on available scientific evidence and strongly supported by expert opinion. Usually<br />
supported by Class II data or a preponderance of Class III evidence.<br />
• Level 3: Supported by available data, but scientific evidence is lacking. Generally supported by Class III data. Useful for<br />
educational purposes and in guiding future clinical research.<br />
1
INTRODUCTION<br />
Blunt abdominal trauma commonly injures the spleen resulting in either irreparable parenchymal<br />
disruption (necessitating removal of the injured organ) or devascularization of varying degrees. Nonoperative<br />
management may avoid <strong>splenectomy</strong>, but can also result in functional asplenia if the<br />
devascularization is extensive or therapeutic embolization of a portion or all of the spleen is required.<br />
Elective <strong>splenectomy</strong> may be indicated for specific primary disease of the spleen. Loss of functional<br />
splenic tissue places such individuals at high risk for infection by encapsulated organisms such as<br />
Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis. Although the<br />
risk of fulminant septicemia or meningitis as a result of infection by such organisms appears to be less in<br />
the adult population (by virtue of prior exposure to these bacteria), overwhelming <strong>post</strong><strong>splenectomy</strong> sepsis<br />
(OPSS) remains a significant concern in the asplenic patient (1).<br />
The incidence of OPSS is estimated to occur in 0.05% to 2% of splenectomized patients (2). It may<br />
develop immediately or as late as 65 years <strong>post</strong><strong>splenectomy</strong> (2-4). Mortality is significant and reported to<br />
be as high as 50% (4,5). OPSS incidence reduction is dependent upon (2,5-7):<br />
1) Prophylactic education of the patient and physician as to its risk and prevention<br />
2) Rapid recognition of the asplenic individual when infection is suspected<br />
Reduced <strong>post</strong>-<strong>splenectomy</strong> levels of opsonins, splenic tuftsin, and immunoglobulin (IgM) (which promote<br />
phagocytosis of particulate matter and bacteria), hamper the body’s ability to clear encapsulated<br />
organisms (4,6,7). Vaccination, to impart immunity against such infections, is commonly performed<br />
despite the absence of Class I or Class II data to support its efficacy. As 50 to 90% of OPSS infections<br />
are secondary to Streptococcus pneumoniae infection, the polyvalent pneumococcal <strong>vaccine</strong> has been<br />
the most commonly administered <strong>post</strong><strong>splenectomy</strong> <strong>vaccine</strong>. In recent years, the meningococcal and<br />
Haemophilus influenzae type b <strong>vaccine</strong>s have also been advocated (2-17).<br />
Timing of <strong>vaccine</strong> administration following <strong>splenectomy</strong> has been the topic of a longstanding debate. Two<br />
major concerns include the patients’ immunogenicity in the perioperative period and the impaired immune<br />
function of the critically ill (2,13,16,17). The patient’s present state of health should be considered prior to<br />
the administration of <strong>post</strong><strong>splenectomy</strong> <strong>vaccine</strong>s. In patients with moderate to severe acute illness,<br />
vaccination should be delayed until the illness has resolved. This minimizes adverse effects of the<br />
<strong>vaccine</strong> which could be more severe in the presence of illness or could confuse the patient’s clinical<br />
picture (such as a <strong>post</strong>-<strong>vaccine</strong> fever) (16).<br />
All of the <strong>vaccine</strong>s cause adverse reactions which are generally self-limiting and resolve 24-72 hours after<br />
<strong>vaccine</strong> administration. The polyvalent pneumococcal <strong>vaccine</strong> causes a transient and self-limited fever<br />
(in 5% of vaccinated patients), as well as pain and redness at the site for 1-2 days. A hypersensitivity<br />
reaction can occur at the injection site of the Haemophilus influenzae type b <strong>vaccine</strong> along with<br />
occasional fever, aches, and malaise. Both meningococcal <strong>vaccine</strong>s can cause headaches, fatigue,<br />
malaise and injection site reactions.<br />
There are two meningococcal <strong>vaccine</strong>s currently on the market: the meningococcal polysaccharide<br />
<strong>vaccine</strong> (Menomune A/C/Y/W-135) and the meningococcal (groups A, C, Y, W-135) polysaccharide<br />
diphtheria toxoid conjugate (Menactra). Both products provide the same level of immunity against<br />
Neisseria meningitidis. Differences between the two products are as follows (29-32):<br />
1) Menactra is for intramuscular injection only, Menomune is administered subcutaneously.<br />
2) Menactra is a conjugated <strong>vaccine</strong> (adding the diphtheria toxoid).<br />
3) Menomune requires revaccination every 3-5 years. Long-term data with Menactra is not yet<br />
available. The manufacturer, Sanofi Pasteur, has data demonstrating adequate levels for up to 3-<br />
5 years (similar to Menomune). Due to on-going duration studies, it is recommended by the<br />
manufacturer that healthcare providers contact Sanofi-Pasteur prior to revaccination in order to<br />
obtain the most current information.<br />
4) Menactra is approved only for use in adolescents and adults between the ages of 11 and 55 (14-<br />
16,25-27) Menactra has applied for a license for use in children ages 2-10 years of age. There is<br />
currently no data on adults > 55 years of age.<br />
2 Approved 1/7/03<br />
Revised 10/17/06
LITERATURE REVIEW<br />
Two Class I studies have demonstrated that the polyvalent pneumococcal <strong>vaccine</strong> results in the highest<br />
antibody titers, for the most common serotypes, when administered 14 days <strong>post</strong><strong>splenectomy</strong> (16,17).<br />
These prospective, randomized trials evaluated the efficacy of the <strong>vaccine</strong> when administered at 1, 7, 14,<br />
and 28 days <strong>post</strong><strong>splenectomy</strong>. As these trials were designed to demonstrate the immunogenicity of the<br />
<strong>vaccine</strong>s and not the prevention of OPSS, they can only be used to advocate timing of vaccination.<br />
In 2004, Landgren et.al. published a prospective study on antibody response to repeated vaccination.<br />
This study included 28 (out of 311) <strong>post</strong>-trauma splenectomized patients. Their results showed that time<br />
between <strong>splenectomy</strong> and first pneumococcal <strong>vaccine</strong> was not associated with pre-vaccination, peak or<br />
follow-up antibody levels. 25 of the 28 trauma patients received their 1 st <strong>vaccine</strong> <strong>post</strong>-<strong>splenectomy</strong>. A<br />
major limitation of this study is that the time from <strong>splenectomy</strong> to first <strong>vaccine</strong> was only documented in<br />
24% of the cases, yet they claimed that timing had no effect (18). Similarly, Grimfors et.al. conducted a<br />
longitudinal study of 173 patients (33 trauma) for three years. Pneumococcal antibody responses<br />
declined to pre-treatment values at three years in all groups. They also found no correlation between the<br />
interval from <strong>splenectomy</strong> to vaccination and response to vaccination. The data to support this<br />
conclusion was not published (19).<br />
Schreiber et.al., published a study in rats in 1998 looking at timing of vaccination and subsequent ability<br />
to survive pneumococcal challenge. There was no difference in ability to survive a pneumococcal<br />
challenge between rats vaccinated on <strong>post</strong>-operative day 1, 7, or 42 (20). In another study, Werner et.al.<br />
looked at the effect of perioperative hypovolemic shock and response to vaccination and found no<br />
difference if splenectomized rats were vaccinated on <strong>post</strong>-operative day 1, 7, or 28. Both of these studies<br />
raise the question of whether delaying vaccination for 14 days as suggested in the Shatz et.al. studies is<br />
necessary (21). Further human studies are needed to address the timing of <strong>post</strong>-<strong>splenectomy</strong> <strong>vaccine</strong>s.<br />
Class II data supports the vaccination of asplenic patients based on studies of the spleen’s role in<br />
immune function and its ability to provide defense against encapsulated organisms (5). Current Center for<br />
Disease Control (CDC) recommendations for <strong>post</strong>-<strong>splenectomy</strong> vaccinations include the polyvalent<br />
pneumococcal (Pneumovax 23), the meningococcal (groups A, C, Y, W-135) polysaccharide diphtheria<br />
toxoid conjugate (Menactra, for patients ages 11-55) or the meningococcal polysaccharide (Menomune<br />
A/C/Y/W-135, ages 55), and the Haemophilus influenzae type b <strong>vaccine</strong>s (Hib TITER) (12-14,25-<br />
28). All three of these <strong>vaccine</strong>s may be administered simultaneously (15).<br />
Revaccination needs have been established by Class II studies of immune antibody levels and efficacy<br />
after initial vaccination (3). Patients receiving the pneumococcal <strong>vaccine</strong> should be revaccinated 5 years<br />
later (28). Patients who receive the meningococcal polysaccharide <strong>vaccine</strong> (Menomune A/C/Y/W-135)<br />
should be revaccinated every 3-5 years (12-14, 26, 27). Patients who receive the meningococcal (groups<br />
A, C, Y, W-135) polysaccharide diphtheria toxoid conjugate <strong>vaccine</strong> (Menactra) probably should be<br />
revaccinated every 3-5 years. However, long-term studies are currently on-going and the manufacturer,<br />
Sanofi-Pasteur, suggests contacting them (1-570-839-7187) for the latest recommendations prior to<br />
revaccination. The Haemophilus influenzae type b <strong>vaccine</strong> does not require revaccination (12-14).<br />
There is no Class I data identifying the appropriate timing for pre-<strong>splenectomy</strong> Haemophilus,<br />
Pneumococcal or Meningococcal vaccination for patients with nonfunctional or diseased spleens.<br />
Vaccination two weeks prior to surgery is commonly practiced, but this is supported only by Class III data<br />
(16, 17). Pre-<strong>splenectomy</strong> vaccination has been demonstrated to induce antibody formation in both<br />
adults and children (18). The types of antibody produced and time to antibody formation (generally 1 to 4<br />
weeks) does vary from patient to patient. (18-20). The antibody titer required to prevent either<br />
pneumococcal carriage or disease is unknown and has been extrapolated from data obtained from the<br />
literature on Haemophilus influenzae titers (21). In the elective <strong>splenectomy</strong> patient, therefore,<br />
vaccination as soon as splenic disease is diagnosed appears prudent to allow time for antibody<br />
production (13). The CDC has outlined recommendations for both initial vaccination in the pediatric<br />
population as well as booster (revaccination) requirements in patients with an anatomically present, but<br />
non-functional spleen (23).<br />
3 Approved 1/7/03<br />
Revised 10/17/06
The CDC recommends that asplenic travelers contact an international health clinic or the CDC<br />
(www.cdc.gov) to obtain information on disease risks within the intended country of travel. Asplenic<br />
travelers should be advised of the increased risk for Meningococcal meningitis and recommendation of<br />
the A and C <strong>vaccine</strong> for all asplenic individuals traveling to sub-Saharan Africa, India, and Nepal.<br />
REFERENCES<br />
1. King H, Schumacher HB. Splenic studies: I. Susceptibility to infection after <strong>splenectomy</strong> in infancy.<br />
Ann Surg 1952; 136(2):239-242.<br />
2. Shatz DV. Vaccination practices among North American trauma surgeons in <strong>splenectomy</strong> for trauma.<br />
J Trauma 2002; 53:950-956.<br />
3. Rutherford EJ, Livengood J, Higginbotham M, et.al. Efficacy and safety of pneumococcal<br />
revaccination after <strong>splenectomy</strong> for trauma. J Trauma 1995; 39:448-452.<br />
4. Dickerman JD. Traumatic asplenia in adults. Arch Surg 1981; 116:361-363.<br />
5. Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin<br />
Microbial Infect 2001; 7:657-60.<br />
6. Waghorn DJ. Overwhelming infection in asplenic patients: current best practice preventative<br />
measures are not be followed. J Clin Pathology 2001; 54:214-218.<br />
7. Brigden ML, Pattulo AL. Prevention and management of overwhelming <strong>post</strong><strong>splenectomy</strong> infection –<br />
an update. Crit Care Med 1999; 27:836-42.<br />
8. Spickett GP, Bulliore J, Wallis J, et.al. Northern region asplenia register – analysis of first two years. J<br />
Clin Pathology 1999; 52:424-429.<br />
9. Williams DN, Bhavjot K. Post<strong>splenectomy</strong> care strategies to decrease the risk of infection. Postgrad<br />
Med 1996: 100: 195-8,201,205.<br />
10. Sumaraju V, Smith LG, Smith SM. Infectious complications in asplenic hosts. Infect Dis Clin N<br />
America 2001; 15:551-65.<br />
11. Styrt B. Infections associated with asplenia: risks, mechanisms, and prevention. Am J Med 1990;<br />
88:33N-42N.<br />
12. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on<br />
Immunization Practices (AICP). MMWR 2000; 49(RR-07):1-3.<br />
13. Prevention and management of infections in patients without a spleen. Clin Micro and Infect 2001;<br />
12:657-680.<br />
14. Recommendations of the Advisory Committee on Immunization Practices (AICP): Use of <strong>vaccine</strong>s<br />
and immune globulins in persons with altered immunocompetence. MMWR 1993; 42(RR-04):1-18.<br />
15. General recommendations on immunization: recommendations of the Advisory Committee on<br />
Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR<br />
2002; 51(RR-2):1-36.<br />
16. Shatz DV, Romero-Steiner S, Elie CM, et.al. Antibody responses in <strong>post</strong><strong>splenectomy</strong> trauma patients<br />
receiving the 23-valent pneumococcal polysaccharide <strong>vaccine</strong> at 14 versus 28 days <strong>post</strong>operatively. J<br />
Trauma 2002; 53:1037-1042.<br />
17. Shatz DV, Schinsky MF, Pais LB, et.al. Antibody responses in <strong>post</strong><strong>splenectomy</strong> trauma patients<br />
receiving the 23-valent pneumococcal polysaccharide <strong>vaccine</strong> at 1 versus 7 versus 14 days after<br />
<strong>splenectomy</strong>. J Trauma 1998; 44(6):760-766.<br />
18. Landgren O, Bjoerkholm M, Konradsen HB, et.al. A prospective study on antibody response to<br />
repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with<br />
special reference to Hodgkin’s lymphoma. J Intern Med 2004;255:644-673.<br />
19. Grimfors G, Soederqvist M, Holm G, et.al. A longitudinal study of class and subclass antibody<br />
response to pneumococcal vaccination in splenectomized individuals with special reference to<br />
Hodgkin’s disease. Eur J Haematol 1990;45:101-108.<br />
20. Schreiber MA, Pusateri AE, Veit BC, et.al. Timing of vaccination does not affect antibody response or<br />
survival after pneumococcal challenge in splenectomized rats. J Trauma 1998;45(4):692-697.<br />
21. Werner AM, Katner HP, Vogel R, et.al. Delayed vaccination dos not improve antibody responses in<br />
splenectomized rats experiencing hypovolemic shock. Am Surg 2001;67(9):834-838.<br />
22. Klinge J, Hammersen G, Scharf J, et.al. Overwhelming <strong>post</strong><strong>splenectomy</strong> infection with <strong>vaccine</strong>-type<br />
Streptococcus pneumoniae in a 12-year-old girl despite vaccination and antibiotic <strong>prophylaxis</strong>.<br />
Infection 1997; 25:368-371.<br />
4 Approved 1/7/03<br />
Revised 10/17/06
23. Wong WY, Overturf GD, Powars DR. Infection caused by Streptococcus pneumoniae in children with<br />
sickle cell disease: epidemiology, immunologic mechanisms, <strong>prophylaxis</strong>, and vaccination. Clin Infect<br />
Dis 192; 14:1124-1136.<br />
24. Siber GR, Gorham C, Martin P, et.al. Antibody response to pretreatment immunization and <strong>post</strong>treatment<br />
boosting with bacterial polysaccharide <strong>vaccine</strong>s in paitents with Hodgkin’s disease. Ann<br />
Intern Med 1986; 104:467-475.<br />
25. Hosea SW, Burch CG, Brown EJ, et.al. Impaired immune response of splenectomized patients to<br />
polyvalent pneumococcal <strong>vaccine</strong>. Lancet 1981; 1:804-807.<br />
26. Cimaz R, Mensi C, D’Angelo E, et.al. Safety and immunogenicity of a conjugate <strong>vaccine</strong> against<br />
Haemophilus influenzae type b in splenectomized and nonsplenectomized patients with Cooley<br />
anemia. J Infect Dis 2001; 183:1819-1821.<br />
27. Jockovich M, Menednhall NP, Sombeck MD, et.al. Long-term complications of laparotomy in<br />
Hodgkin’s disease. Ann Surg 1994; 219:615-621.<br />
28. CDC: Preventing pneumococcal disease among infants and young children. MMWR 2000; 49:1-38.<br />
29. Meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate <strong>vaccine</strong>:<br />
Menactra ® . Package Insert. Sanofi Pasteur. 2005 Dec: 1-10. www.menactra.com [Accessed 08-28-<br />
2006].<br />
30. Meningococcal polysaccharide <strong>vaccine</strong>. Drug Shortage Bulletin. ASHP. 2006 May 22. www.ashp.org<br />
[Accessed 08-28-2006].<br />
31. Meningococcal conjugate <strong>vaccine</strong> (MCV-4): AICP Recommendation. National Infection Prevention.<br />
CDC 2006. www.cdc.gov/nip/<strong>vaccine</strong>/mening/mcv4/mcv4_aicp.htm [Accessed 08-28-2006].<br />
32. Revisions to the general recommendations on immunizations. CDC 2006 Sept. www.cdc.gov<br />
[Accessed 08-28-2006]. PowerPoint presentation.<br />
5 Approved 1/7/03<br />
Revised 10/17/06
POSTSPLENECTOMY PATIENT INFORMATION SHEET<br />
Name:<br />
Splenectomy (splee-nek-tuh-mee) is the name of the operation that was done to remove your spleen.<br />
The spleen is a fist-sized organ located in the upper left side of your abdomen (belly). The spleen helps<br />
you fight infections, get rid of old or damaged red blood cells, and store blood for your body. Because of<br />
either disease or damage to your spleen, it had be removed. You can live without a spleen, but you may<br />
be at a higher risk for certain types of blood infection. To help you fight these infections in the future, you<br />
have been given the following immunizations (shots):<br />
□ Pneumococcal <strong>vaccine</strong>, polyvalent (Pneumovax 23)<br />
**Revaccinate every 6 years**<br />
□ Age > 55: Meningococcal polysaccharide <strong>vaccine</strong><br />
(Menomune A/C/Y/W-135) **Revaccinate every 3-5 years**<br />
□ Age 16-55: Meningococcal polysaccharide/diphtheria toxoid conjugate <strong>vaccine</strong><br />
(Menactra A/C/Y/W-135) **May need revaccination every 3-5 years**<br />
□ Haemophilus influenzae type b conjugate <strong>vaccine</strong><br />
**No revaccination needed**<br />
Date:<br />
Date:<br />
Date:<br />
Date:<br />
It is important that you go and see a doctor IMMEDIATELY if you have any of the following symptoms:<br />
• Fever<br />
• Chills<br />
• Abdominal pain<br />
• Skin rash, swelling, redness, or infection<br />
• Diarrhea<br />
• Achy or weak feeling<br />
• Cough<br />
• Vomiting<br />
These are signs that you may have an infection. Without your spleen, a small or minor infection my<br />
become very serious and your doctor needs to examine you and possibly start antibiotics to help your<br />
body fight the infection. Always check with your doctor before any dental or invasive procedures, as you<br />
may need to take antibiotics before the procedure.<br />
The effect of the <strong>vaccine</strong>s in preventing infection varies from patient to patient and depends on the<br />
strength of your immune system when the <strong>vaccine</strong>s were given. You will need to be re-immunized (have<br />
the shots again) approximately every 5 years for the rest of your life. You should make sure that your<br />
doctor has a copy of this information sheet so that they can help remind you when it is time to be reimmunized.<br />
If you or your doctor have any questions about the above information, you should contact your<br />
surgeon:<br />
Surgeon’s Name:<br />
Surgeon’s Phone Number:<br />
6 Approved 1/7/03<br />
Revised 10/17/06