1u7kf0g
1u7kf0g
1u7kf0g
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
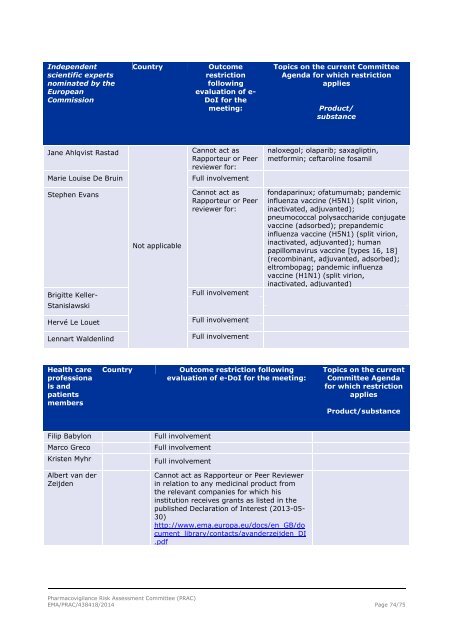
Independent<br />
scientific experts<br />
nominated by the<br />
European<br />
Commission<br />
Country<br />
Outcome<br />
restriction<br />
following<br />
evaluation of e-<br />
DoI for the<br />
meeting:<br />
Topics on the current Committee<br />
Agenda for which restriction<br />
applies<br />
Product/<br />
substance<br />
Jane Ahlqvist Rastad<br />
Cannot act as<br />
Rapporteur or Peer<br />
reviewer for:<br />
naloxegol; olaparib; saxagliptin,<br />
metformin; ceftaroline fosamil<br />
Marie Louise De Bruin<br />
Full involvement<br />
Stephen Evans<br />
Brigitte Keller-<br />
Stanislawski<br />
Not applicable<br />
Cannot act as<br />
Rapporteur or Peer<br />
reviewer for:<br />
Full involvement<br />
fondaparinux; ofatumumab; pandemic<br />
influenza vaccine (H5N1) (split virion,<br />
inactivated, adjuvanted);<br />
pneumococcal polysaccharide conjugate<br />
vaccine (adsorbed); prepandemic<br />
influenza vaccine (H5N1) (split virion,<br />
inactivated, adjuvanted); human<br />
papillomavirus vaccine [types 16, 18]<br />
(recombinant, adjuvanted, adsorbed);<br />
eltrombopag; pandemic influenza<br />
vaccine (H1N1) (split virion,<br />
inactivated, adjuvanted)<br />
Hervé Le Louet<br />
Full involvement<br />
Lennart Waldenlind<br />
Full involvement<br />
Health care<br />
professiona<br />
ls and<br />
patients<br />
members<br />
Country<br />
Outcome restriction following<br />
evaluation of e-DoI for the meeting:<br />
Topics on the current<br />
Committee Agenda<br />
for which restriction<br />
applies<br />
Product/substance<br />
Filip Babylon<br />
Full involvement<br />
Marco Greco<br />
Full involvement<br />
Kristen Myhr<br />
Full involvement<br />
Albert van der<br />
Zeijden<br />
Cannot act as Rapporteur or Peer Reviewer<br />
in relation to any medicinal product from<br />
the relevant companies for which his<br />
institution receives grants as listed in the<br />
published Declaration of Interest (2013-05-<br />
30)<br />
http://www.ema.europa.eu/docs/en_GB/do<br />
cument_library/contacts/avanderzeijden_DI<br />
.pdf<br />
Pharmacovigilance Risk Assessment Committee (PRAC)<br />
EMA/PRAC/438418/2014 Page 74/75