You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
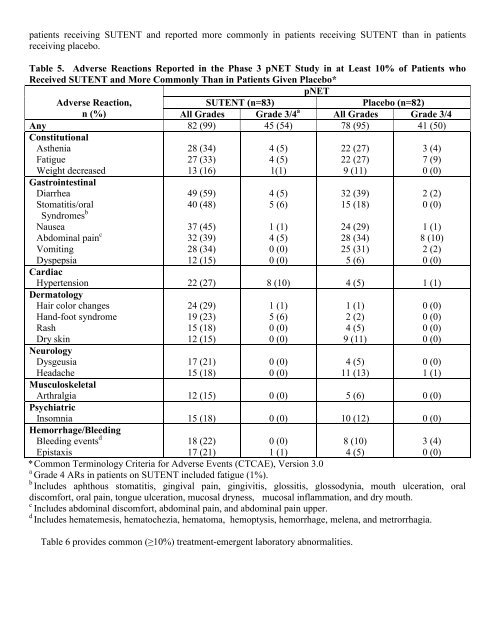
patients receiving SUTENT and reported more commonly in patients receiving SUTENT than in patients<br />
receiving placebo.<br />
Table 5. Adverse Reactions Reported in the Phase 3 pNET Study in at Least 10% of Patients who<br />
Received SUTENT and More Commonly Than in Patients Given Placebo*<br />
pNET<br />
Adverse Reaction,<br />
SUTENT (n=83)<br />
Placebo (n=82)<br />
n (%)<br />
All Grades Grade 3/4 a All Grades Grade 3/4<br />
Any 82 (99) 45 (54) 78 (95) 41 (50)<br />
Constitutional<br />
Asthenia<br />
Fatigue<br />
Weight decreased<br />
28 (34)<br />
27 (33)<br />
13 (16)<br />
4 (5)<br />
4 (5)<br />
1(1)<br />
22 (27)<br />
22 (27)<br />
9 (11)<br />
3 (4)<br />
7 (9)<br />
0 (0)<br />
Gastrointestinal<br />
Diarrhea<br />
Stomatitis/oral<br />
Syndromes b<br />
Nausea<br />
Abdominal pain c<br />
Vomiting<br />
Dyspepsia<br />
49 (59)<br />
40 (48)<br />
37 (45)<br />
32 (39)<br />
28 (34)<br />
12 (15)<br />
4 (5)<br />
5 (6)<br />
1 (1)<br />
4 (5)<br />
0 (0)<br />
0 (0)<br />
32 (39)<br />
15 (18)<br />
24 (29)<br />
28 (34)<br />
25 (31)<br />
5 (6)<br />
2 (2)<br />
0 (0)<br />
1 (1)<br />
8 (10)<br />
2 (2)<br />
0 (0)<br />
Cardiac<br />
Hypertension 22 (27) 8 (10) 4 (5) 1 (1)<br />
Dermatology<br />
Hair color changes<br />
Hand-foot syndrome<br />
Rash<br />
Dry skin<br />
Neurology<br />
Dysgeusia<br />
Headache<br />
24 (29)<br />
19 (23)<br />
15 (18)<br />
12 (15)<br />
17 (21)<br />
15 (18)<br />
1 (1)<br />
5 (6)<br />
0 (0)<br />
0 (0)<br />
0 (0)<br />
0 (0)<br />
1 (1)<br />
2 (2)<br />
4 (5)<br />
9 (11)<br />
4 (5)<br />
11 (13)<br />
0 (0)<br />
0 (0)<br />
0 (0)<br />
0 (0)<br />
0 (0)<br />
1 (1)<br />
Musculoskeletal<br />
Arthralgia 12 (15) 0 (0) 5 (6) 0 (0)<br />
Psychiatric<br />
Insomnia 15 (18) 0 (0) 10 (12) 0 (0)<br />
Hemorrhage/Bleeding<br />
Bleeding events d<br />
Epistaxis<br />
18 (22)<br />
0 (0)<br />
8 (10)<br />
3 (4)<br />
17 (21)<br />
1 (1)<br />
4 (5)<br />
0 (0)<br />
*Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0<br />
a Grade 4 ARs in patients on SUTENT included fatigue (1%).<br />
b Includes aphthous stomatitis, gingival pain, gingivitis, glossitis, glossodynia, mouth ulceration, oral<br />
discomfort, oral pain, tongue ulceration, mucosal dryness, mucosal inflammation, and dry mouth.<br />
c Includes abdominal discomfort, abdominal pain, and abdominal pain upper.<br />
d Includes hematemesis, hematochezia, hematoma, hemoptysis, hemorrhage, melena, and metrorrhagia.<br />
Table 6 provides common (≥10%) treatment-emergent laboratory abnormalities.