Unit 4: Chemical Bonding and Molecular Structure Chapter 6 Notes ...
Unit 4: Chemical Bonding and Molecular Structure Chapter 6 Notes ...
Unit 4: Chemical Bonding and Molecular Structure Chapter 6 Notes ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Unit</strong> 4: <strong>Chemical</strong> <strong>Bonding</strong> <strong>and</strong> <strong>Molecular</strong> <strong>Structure</strong><br />
<strong>Chapter</strong> 6 <strong>Notes</strong>: <strong>Chemical</strong> <strong>Bonding</strong><br />
6.1: Introduction to <strong>Chemical</strong> <strong>Bonding</strong> <br />
I. <strong>Chemical</strong> Bonds <br />
<strong>Chemical</strong> Bond: attractive force between atoms or ions that binds them <br />
together as a unit <br />
** Concept: Why do most atoms form chemical bonds Independent particles are at <br />
relatively high potential energy. (Most atoms are less stable existing by themselves.) <br />
Nature favors arrangements in which potential energy is minimized-‐ <br />
1. decreasing potential energy <br />
2. creating more stable arrangements of matter. <br />
II. Types of <strong>Chemical</strong> Bonds: <br />
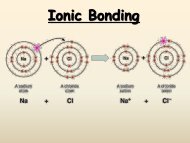
1. Ionic bonding: <strong>Chemical</strong> bonding that results from the electrical attraction <br />
between positively charged cations <strong>and</strong> negatively charged anions. (In purely <br />
ionic bonding, electrons are transferred from one ion to another.) <br />
2. Covalent bonding: Atoms joined by covalent bonding share electrons. Covalent <br />
bonding results from the sharing of electron pairs between two atoms. (In <br />
purely covalent bonding, the shared electrons are “owned” equally by the two <br />
bonded atoms.) <br />
3. Metallic bonding: the chemical bonding that results from the attraction between <br />
metal atoms <strong>and</strong> the surrounding sea of electrons <br />
III. Ionic or Covalent <br />
Using Electronegativity: a measure of the ability of an atom in a chemical compound <br />
to attract electrons. The degree to which bonding between atoms of two elements is <br />
ionic or covalent can be estimated by calculating the difference in the elements’ <br />
electronegativities. (END-‐ electronegativity difference) <br />
Ionic: <strong>Bonding</strong> between atoms with an electronegativity difference of greater than 1.7 <br />
Example: Fluorine (4.0) <strong>and</strong> Cesium (0.7) <br />
4.0 – 0.7 = 3.3 will be an ionic bond. <br />
Covalent: <strong>Bonding</strong> between atoms with an electronegativity difference of 1.7 or less <br />
will be a covalent bond. <br />
IV. Two types of Covalent bonds: <br />
Covalent bonds will be either polar-‐covalent or non-‐polar covalent. <br />
A. Non-‐polar covalent bonds: a covalent bond in which the bonding electrons are <br />
shared equally by the bonded atoms, resulting in a balanced distribution of electrical <br />
charge. <br />
Example: <strong>Bonding</strong> between atoms of the same element is completely covalent. <br />
(The electronegativity difference will equal zero.) Generally, electronegativity <br />
differences between 0 <strong>and</strong> 0.3 are considered non-‐polar covalent bonds.
B. Polar-‐covalent bonds: a covalent bond in which the bonded atoms have an <br />
unequal attraction for the shared electrons. Generally, electronegativity differences <br />
between 0.3 <strong>and</strong> 1.7 are classified as polar. <br />
Example: The electronegativity difference between Chlorine <strong>and</strong> hydrogen is <br />
3.0 – 2.1 = 0.9 indicating a polar-‐covalent bond. <br />
The electrons in this bond are closer to the more-‐electronegative chlorine atom than to <br />
the hydrogen atom. Therefore, the chlorine end of the bond has a partial negative <br />
charge, indicated by the δ -‐ . The hydrogen end of the bond then has an equal partial <br />
positive charge, δ + . <br />
δ + δ -<br />
H Cl<br />
** Concept: Why is most chemical bonding neither purely ionic nor purely covalent <br />
<strong>Bonding</strong> between different elements is rarely purely ionic or purely covalent. It <br />
usually falls somewhere between these two extremes, depending on how strongly the <br />
atoms of each element attract electrons. <br />
6.2: Covalent <strong>Bonding</strong> <strong>and</strong> <strong>Molecular</strong> Compounds <br />
I. Important Definitions: <br />
A. Molecule: a neutral group of atoms that are held together by covalent bonds <br />
B. <strong>Molecular</strong> compound: a chemical compound whose simplest units are molecules <br />
C. <strong>Chemical</strong> formula: indicates the relative numbers of atoms of each kind of a <br />
chemical compound by using atomic symbols <strong>and</strong> numerical subscripts <br />
D. <strong>Molecular</strong> formula: shows the tpes <strong>and</strong> numbers of atoms combined in a single <br />
molecule of a molecular compound <br />
II. Nature of the Covalent Bond <br />
A. A bond between two nonmetals. Nonmetal to nonmetal <br />
B. electrons are shared (the unpaired valence electrons) <br />
C. Very strong bond <br />
1. strong intramolecular forces (force of attraction within molecule)
2. can not be broken up by dissolving in water or melting, so never conduct <br />
electricity (exception later in the year) <br />
3. bond length <strong>and</strong> bond energy vary depending on the types of atoms bonded <br />
D. Intermolecular forces (force of attraction between molecules) <br />
1. much weaker than forces between ionic formula units <br />
2. compared to ionic compounds: <br />
a. lower melting points <strong>and</strong> boiling points <br />
b. do not conduct electricity <br />
III. The Octet Rule <br />
A. <strong>Chemical</strong> compounds tend to form so that each atom, by gaining, losing, or sharing <br />
electrons, has an octet of electrons in its highest occupied energy level. <br />
B. Covalent compounds tend to form so that each atom, by sharing electrons, <br />
completes an octet of electrons in its highest occupied energy level. <br />
C. Exceptions to the octet rule: <br />
1. Hydrogen: forms bonds in which it’s surrounded by two electrons <br />
2. Boron: (3 valence electrons) forms bonds in which it’s surrounded by six <br />
electrons <br />
IV. Electron-‐Dot Notation <br />
A. Definition: an electron-‐configuration notation in which only the valence electrons <br />
of an atom of a particular element are shown, indicated by dots placed around the <br />
element’s symbol. The inner-‐shell electrons are not shown. <br />
V. Lewis <strong>Structure</strong>s: <br />
A. Electron dot notation can also be used to represent molecules. <br />
B. Unshared Pairs of electrons (Lone Pairs) <br />
1. A pair of electrons that is not involved in bonding <strong>and</strong> that belongs <br />
exclusively to one atom <br />
2. represented by two dots <br />
C. Shared pairs of electrons <br />
1. Electrons pairs involved in covalent bonds (shared) <br />
2. represented by dash
D. Drawing Lewis <strong>Structure</strong>s (example: trichloromethane, CHCl3) <br />
1. Determine the type <strong>and</strong> number of atoms in the molecule <br />
1 x C, 1 x H, 3 x Cl <br />
2. Determine the total number of valence electrons to be accounted for <br />
C 1 x 4 e -‐ = 4e -‐ <br />
H 1 x 1 e -‐ = 1e -‐ <br />
Cl 3 x 7 e -‐ = 21 e -‐ <br />
Total: <br />
26 e -‐ <br />
3. Arrange atoms to form a skeleton structure for the molecule. If carbon is <br />
present, it is the central atom. Otherwise the least elecronegative element <br />
atom is central (except for hydrogen, which is never central). Then connect <br />
the atoms by electron-‐ pair bonds. <br />
4. Add unshared pairs of electrons so that each nonmetal is surrounded by <br />
eight electrons (except hydrogen-‐ just two)—octet rule. <br />
5. Count the electrons in the structure to be sure that the number of valence <br />
electrons used equals the number available. <br />
VI. Multiple Covalent Bonds <br />
A. Double Bonds <br />
1. A covalent bond produced by the shring of two pairs of electrons between <br />
two atoms <br />
2. Higher bond energy <strong>and</strong> shorter bond length than single bonds <br />
B. Triple Bonds <br />
1. A covalent bond produced by the sharing of three pairs of electrons between <br />
two atoms <br />
2. Higher bond energy <strong>and</strong> shorter bond length than single bonds <br />
VII. Resonance <strong>Structure</strong>s
A. Resonance <strong>Structure</strong>: bonding in molecules or ions that cannot be correctly <br />
represented by a single Lewis <strong>Structure</strong> <br />
B. To indicate resonance, a double-‐headed arrow is placed between a molecule’s <br />
resonance structures. <br />
6.3 Ionic <strong>Bonding</strong> <strong>and</strong> Ionic Compounds <br />
I. Important Definitions <br />
A. Ionic compound: composed of positive <strong>and</strong> negative ions that are combined so that <br />
the numbers of positive <strong>and</strong> negative charges are equal <br />
B. Formula unit: the simplest whole number ratio of atoms from which an ionic <br />
compound’s formula can be established <br />
C. Crystal lattice structure: an orderly arrangement of ions that minimizes potential <br />
energy <br />
D. Lattice energy: the energy released when one mole of an ionic crystalline <br />
compound is formed from gaseous ions <br />
E. Polyatomic ions: a charged group of covalently bonded atoms <br />
II. Formation of Ionic Compounds <br />
A. A bond between a positive ion (cation) <strong>and</strong> a negative ion (anion) <br />
B. Metal ions bonded to nonmetal ions (metal to nonmetal) <br />
C. Electron Configuration Changes <br />
1. Electrons are trasferred from the highest energy level of one atom to the <br />
highest energy level of a second atom, creating stable ions. <br />
2. Opositely charged ions come together in a ratio that produces a net charge <br />
of zero. <br />
a. Na = 3s 1 Cl = 3s 2 3p 5 <br />
b. Na +1 = 2s 2 2p 6 Cl -‐1 = 3s 2 3p 6 <br />
c. NaCl <br />
III. Polyatomic ions <br />
A. charged group of covalently bonded atoms <br />
B. Mostly anions, one main exception: NH4 +1 <br />
C. Lewis structures: <br />
1. Net charge equals charge of the ion <br />
2. Written in brackets to show that the group as a whole has a charge. <br />
IV. Comparing Ionic <strong>and</strong> Covalent Compounds <br />
Ionic Compounds Covalent Compound <br />
<strong>Structure</strong> Crystal lattice Molecule <br />
Melting Point High Usually low <br />
Boiling Point High Lower <br />
Electrical Conductivity Yes, when dissolved, or <br />
molten (not solid) <br />
No, usually(exception noted <br />
later) <br />
Solubility in water Yes Polar = yes, Non-‐polar = no
6.4 Metallic <strong>Bonding</strong> <br />
I. The Metallic Bond Model <br />
A. Metallic <strong>Bonding</strong> <br />
1. The chemical bonding that results from the attraction between metal atoms <br />
<strong>and</strong> the surrounding sea of electrons <br />
B. Electron Delocalization in Metals <br />
1. Vacant p <strong>and</strong> d orbitals in metal's outer energy levels overlap, <strong>and</strong> allow <br />
outer electrons to move freely throughout the metal <br />
2. Valence electrons do not belong to any one atom <br />
II. Metallic Properties <br />
A. Metals are good conductors of heat <strong>and</strong> light <br />
B. Metals are shiny <br />
1. Narrow range of energy differences between orbitals allows electrons to be <br />
easily excited, <strong>and</strong> emit light upon returning to a lower energy level <br />
C. Metals are Malleable <br />
1. Can be hammered into thin sheets <br />
D. Metals are ductile <br />
1. Ability to be drawn into wire <br />
a. Metallic bonding is the same in all directions, so metals tend not to be <br />
brittle <br />
E. Metals atoms organized in compact, orderly crystalline patterns <br />
F. Different metallic elements (<strong>and</strong> carbon) can be mixed to form alloys <br />
1. Sterling silver <br />
a. Ag = 92.5%, Cu = 7.5% <br />
2. Brass <br />
a. Cu = 60%, Zn = 40% <br />
6.5 <strong>Molecular</strong> Geometry <br />
A. VSEPR theory <br />
1. valence-‐shell, electron-‐pair repulsion <br />
2. states that repulsion between the sets of valence-‐level electrons surrounding <br />
an atom causes these sets to be oriented as far apart as possible <br />
3. shared pairs of electrons will be as far apart from each other as possible <br />
B. Unshared Electron Pairs <br />
1. Lone pairs (unshared electron pairs) occupies space around the atom just as <br />
bonding pairs do. <br />
2. Lone pairs have a relatively greater effect on geometry than shared pairs <br />
(Have greater repusive forces & tend to compress the angles between <br />
bonding pairs) <br />
C. Double <strong>and</strong> triple bonds are treated in the same way as single bonds <br />
D. Polyatomic ions are treated similarly to molecules.
E. <strong>Molecular</strong> Polarity <br />
1. The uneven distribution of molecular charge <br />
2. A dipole is created by equal but opposite charges that are separated by a <br />
short distance. <br />
3. The direction of the dipole is from the positive pole to its negative pole <br />
4. A dipole is represented by an arrow with a head pointing toward the negatie <br />
pole <strong>and</strong> a crossed tail situated at the positive pole.