Introduction to Force Fields
Introduction to Force Fields
Introduction to Force Fields
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chemistry 380.37<br />
May 2013<br />
Dr. Jean M. Standard<br />
May 14, 2013<br />
Molecular Mechanics – <strong>Introduction</strong> <strong>to</strong> <strong>Force</strong> <strong>Fields</strong><br />
Molecular mechanics is a method in which an a<strong>to</strong>m is treated as a single unit consisting of the electrons and the<br />
nucleus; that is, the electrons are not treated explicitly. A bond between two a<strong>to</strong>ms is described as two masses<br />
joined by a spring. In molecular mechanics, the energy of a molecule is determined from an empirical function<br />
called a force field that depends upon the coordinates of the a<strong>to</strong>ms that comprise the molecule.<br />
The Molecular Mechanics <strong>Force</strong> Field<br />
A typical molecular mechanics force field consists of terms that describe bond stretching, angle bending, <strong>to</strong>rsional<br />
motion, nonbonded interactions, and electrostatic interactions,<br />
U =<br />
∑ U s + U b<br />
bonds angles<br />
∑ + ∑ U t + ∑ U nb + ∑ U el . (1)<br />
<strong>to</strong>rsions<br />
nonbond<br />
interactions<br />
charge pairs<br />
Evaluation of the force field for a particular molecular geometry provides the steric or strain energy, U. The force<br />
field energy is sometimes denoted by V <strong>to</strong> represent that it acts as the potential energy in classical mechanics<br />
€<br />
simulations.<br />
The Stretching Energy<br />
The stretching energy U s describes the energy of a bond between two connected a<strong>to</strong>ms. A typical functional form<br />
for the stretching energy involves a harmonic function,<br />
€<br />
U s = 1 2 k s,AB ( r AB − r AB,eq ) 2 , (2)<br />
€<br />
where r AB is the instantaneous bond length between a<strong>to</strong>ms A and B, r AB,eq is the equilibrium (or strain-free) bond<br />
length, and k s,AB is the stretching force<br />
€<br />
constant for the A-B bond. The magnitude of the force constant depends<br />
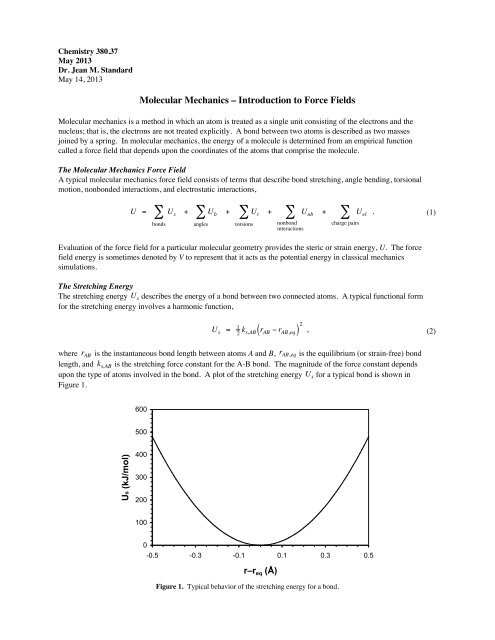
upon the type of a<strong>to</strong>ms involved in the bond. A plot of the stretching energy U s for a typical bond is shown in<br />
Figure 1.<br />
€<br />
€<br />
600<br />
€<br />
500<br />
Us (kJ/mol)<br />
400<br />
300<br />
200<br />
100<br />
0<br />
-0.5 -0.3 -0.1 0.1 0.3 0.5<br />
r–r eq (Å)<br />
Figure 1. Typical behavior of the stretching energy for a bond.
2<br />
In some force fields (such as MMFF94), cubic and/or quartic corrections may be included <strong>to</strong> account for the<br />
anharmonicity of the stretching potential. In such cases, the stretching energy takes a more complicated form,<br />
U s = 1 2 k s,AB r AB − r AB,eq<br />
Here, the cubic stretching force constant is<br />
( ) 2 + 1 2 k (3)<br />
s,AB ( r AB − r AB,eq ) 3 + 1 2 k (4 )<br />
s,AB ( r AB − r AB,eq ) 4 . (3)<br />
(3)<br />
k s,AB<br />
and the quartic stretching force constant is<br />
(4)<br />
k s,AB .<br />
€<br />
The Bending Energy<br />
The bending energy U b describes the energy corresponding <strong>to</strong> variations of the angle between two bonds. A typical<br />
€<br />
€<br />
function for the bending energy is<br />
€<br />
U b = k b,ABC ( θ ABC −θ ABC,eq ) 2 , (4)<br />
where θ is the instantaneous bond angle, θ ABC,eq is the equilibrium bond angle, and k b,ABC is the bending force<br />
constant for angle A-B-C. A plot of<br />
€<br />
the bending energy for a typical bond angle is shown in Figure 2.<br />
€<br />
2.5<br />
€<br />
€<br />
2.0<br />
Ub (kJ/mol)<br />
1.5<br />
1.0<br />
0.5<br />
0.0<br />
-10.0 -5.0 0.0 5.0 10.0<br />
θ−θ eq (deg)<br />
Figure 2. Typical behavior of the bending energy for a bond angle.<br />
In some force fields (including MMFF94), cubic and/or quartic corrections are added <strong>to</strong> the bending potential <strong>to</strong><br />
improve the accuracy and transferability of parameters.<br />
The Torsional Energy<br />
The <strong>to</strong>rsional energy U t is often described by the function<br />
€<br />
V t = V 1<br />
2<br />
( 1+ cosω ) + V 2<br />
2<br />
( 1+ cos 2ω ) + V 3<br />
2<br />
( 1+ cos 3ω ) + … , (5)<br />
where ω is the <strong>to</strong>rsional angle, and V 1 , V 2 , and V 3 are empirical constants. A typical plot for the <strong>to</strong>rsional energy<br />
of ethane is shown in Figure 3.<br />
€<br />
€<br />
€<br />
€<br />
€
3<br />
1.4<br />
1.2<br />
1.0<br />
Ut (kJ/mol)<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0.0<br />
0 60 120 180 240 300 360<br />
ω (degrees)<br />
Figure 3. Typical behavior of the <strong>to</strong>rsional energy for a rotation about the C-C bond in ethane.<br />
In this case, minima occur when the hydrogens on the adjacent carbons of ethane are staggered, and maxima occur<br />
when the hydrogens are eclipsed.<br />
The Non-bonded Interaction Energy<br />
Another important term in a general molecular mechanics force field is the nonbonded interaction energy, or van der<br />
Waals interaction energy. The non-bonded interaction energy U nb,AB between two nonbonded a<strong>to</strong>ms A and B is<br />
usually described by a Lennard-Jones 6-12 potential,<br />
U nb,AB € = − a AB<br />
+ b AB<br />
6 12 , (6)<br />
where a AB and b AB are empirical parameters for a<strong>to</strong>ms A and B and r AB is the distance between a<strong>to</strong>ms A and B. A<br />
typical plot of the non-bonded interaction<br />
€<br />
energy between two a<strong>to</strong>ms is shown in Figure 4.<br />
r AB<br />
r AB<br />
€<br />
€<br />
25<br />
€<br />
20<br />
15<br />
Unb (kJ/mol)<br />
10<br />
5<br />
0<br />
-5<br />
-10<br />
1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 4.0<br />
r AB (Å)<br />
Figure 4. Typical behavior of the non-bonded interaction energy.
4<br />
The Electrostatic Energy<br />
The Coulomb interaction potential between two charged particles is<br />
U el =<br />
q A q B<br />
4πε 0 r AB<br />
, (7)<br />
€<br />
where q A and q B are the charges on particles A and B and r AB is the distance between a<strong>to</strong>ms A and B. The<br />
attractive interaction between opposite charges in shown in Figure 5(a) and the repulsive interaction between like<br />
€<br />
charges is shown in Figure 5(b).<br />
€<br />
€<br />
Electrostatic Energy<br />
Electrostatic Energy<br />
0 2 4 6 8 10<br />
r AB (Å)<br />
0 2 4 6 8 10<br />
r AB (Å)<br />
(a) (b)<br />
Figure 5. Typical behavior of the electrostatic energy for (a) opposite charges and (b) like charges.<br />
Determination of <strong>Force</strong> Field Parameters<br />
The parameters that make up the force field generally are derived from experimental data. This can be done in many<br />
different ways; thus, there are many different force fields available in the literature. Some have been constructed <strong>to</strong><br />
deal with small organic molecules, others have been generated <strong>to</strong> focus especially on biomolecules, and others are of<br />
general utility.<br />
Sample <strong>Force</strong> Field Parameters<br />
Molecular mechanics (MM) calculations rely on force fields constructed from empirical data. The force field<br />
parameters are obtained from experimental or quantum mechanical results. Shown in Tables 1 and 2 are some<br />
example bond stretching and angle bending force field parameters. These parameters are from the MM3 force field.<br />
Table 1. Example bond stretching parameters, MM3 force field<br />
Bond type<br />
r AB,eq<br />
k s,AB<br />
(Å)<br />
(kJ mol –1 Å –2 )<br />
Csp 3 –Csp 3 €<br />
1.523<br />
€<br />
1330<br />
Csp 3 –Csp 2 1.497 1330<br />
Csp 2 =Csp 2 1.337 2890<br />
Csp 2 =O 1.208 3250<br />
Csp 3 –Nsp 3 1.438 1540<br />
Note that the stretching force constants for double bonds are in general larger than the stretching force constants for<br />
single bonds.
5<br />
Table 2. Example angle bending parameters, MM3 force field<br />
Angle type<br />
θ ABC,eq<br />
k b,ABC<br />
(deg.)<br />
(kJ mol –1 deg –2 )<br />
Csp 3 –Csp 3 –Csp 3 €<br />
109.47<br />
€<br />
0.041<br />
Csp 3 –Csp 3 –H 109.47 0.033<br />
H–Csp 3 –H 109.47 0.029<br />
Csp 3 –Csp 2 –Csp 3 117.2 0.041<br />
Csp 3 –Csp 2 =Csp 2 121.4 0.051<br />
Csp 3 –Csp 2 =O 122.5 0.042<br />
Here, one of the most important things <strong>to</strong> note is that the angle bending force constants are much smaller than the<br />
bond stretching force constants. Also, note that even for a tetrahedral center (such as at an sp 3 -hybridized carbon),<br />
the angle bending force constants depend upon the end a<strong>to</strong>ms. Because of the smaller H a<strong>to</strong>ms one the ends, an H-<br />
C-H force constant will be smaller than a C-C-C force constant.