Register Early and Save up to $300 (See page ... - IBC Life Sciences
Register Early and Save up to $300 (See page ... - IBC Life Sciences
Register Early and Save up to $300 (See page ... - IBC Life Sciences
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
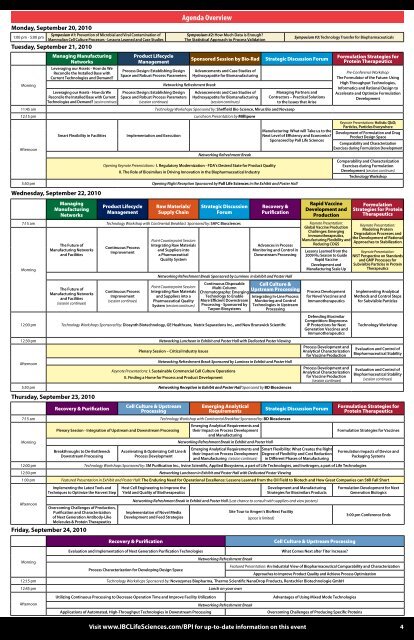
Monday, September 20, 2010 <br />
1:00 pm - 5:00 pm<br />
Symposium #1: Prevention of Microbial <strong>and</strong> Viral Contamination of<br />
Mammalian Cell Culture Processes - Lessons Learned <strong>and</strong> Case Studies<br />
Tuesday, September 21, 2010 <br />
Morning<br />
Managing Manufacturing<br />
Networks<br />
Leveraging our Assets - How do We<br />
Reconcile the Installed Base with<br />
Current Technologies <strong>and</strong> Dem<strong>and</strong><br />
Leveraging our Assets - How do We<br />
Reconcile the Installed Base with Current<br />
Technologies <strong>and</strong> Dem<strong>and</strong> (session continues)<br />
Product <strong>Life</strong>cycle<br />
Management<br />
Process Design: Establishing Design<br />
Space <strong>and</strong> Robust Process Parameters<br />
Process Design: Establishing Design<br />
Space <strong>and</strong> Robust Process Parameters<br />
(session continues)<br />
Agenda Overview<br />
Symposium #2: How Much Data is Enough<br />
The Statistical Approach <strong>to</strong> Process Validation<br />
Networking Refreshment Break<br />
Sponsored Session by Bio-Rad Strategic Discussion Forum<br />
Advancements <strong>and</strong> Case Studies of<br />
Hydroxyapatite for Biomanufacturing<br />
Advancements <strong>and</strong> Case Studies of<br />
Hydroxyapatite for Biomanufacturing<br />
(session continues)<br />
11:45 am Technology Workshops Sponsored by: Sheffield Bio-Science, Mirus Bio <strong>and</strong> Novasep<br />
12:15 pm Luncheon Presentation by Millipore<br />
Afternoon<br />
Smart Flexibility in Facilities<br />
Implementation <strong>and</strong> Execution<br />
Networking Refreshment Break<br />
Opening Keynote Presentations: I. Regula<strong>to</strong>ry Modernization - FDA's Desired State for Product Quality<br />
II. The Role of Biosimilars in Driving Innovation in the Biopharmaceutical Industry<br />
Symposium #3: Technology Transfer for Biopharmaceuticals<br />
Managing Partners <strong>and</strong><br />
Contrac<strong>to</strong>rs – Practical Solutions<br />
<strong>to</strong> the Issues that Arise<br />
Manufacturing: What will Take us <strong>to</strong> the<br />
Next Level of Efficiency <strong>and</strong> Economics<br />
Sponsored by Pall <strong>Life</strong> <strong>Sciences</strong><br />
5:30 pm Opening Night Reception Sponsored by Pall <strong>Life</strong> <strong>Sciences</strong> in the Exhibit <strong>and</strong> Poster Hall<br />
Wednesday, September 22, 2010 <br />
Managing<br />
Manufacturing<br />
Networks<br />
Product <strong>Life</strong>cycle<br />
Management<br />
Raw Materials/<br />
S<strong>up</strong>ply Chain<br />
Strategic Discussion<br />
Forum<br />
Recovery &<br />
Purification<br />
Rapid Vaccine<br />
Development <strong>and</strong><br />
Production<br />
7:15 am Technology Workshop with Continental Breakfast Sponsored by: SAFC Biosciences Keynote Presentation:<br />
Global Vaccine Production<br />
Challenges: Emerging<br />
Morning<br />
The Future of<br />
Manufacturing Networks<br />
<strong>and</strong> Facilities<br />
The Future of<br />
Manufacturing Networks<br />
<strong>and</strong> Facilities<br />
(session continues)<br />
Continuous Process<br />
Improvement<br />
Continuous Process<br />
Improvement<br />
(session continues)<br />
Point Counterpoint Session:<br />
Integrating Raw Materials<br />
<strong>and</strong> S<strong>up</strong>pliers in<strong>to</strong><br />
a Pharmaceutical<br />
Quality System<br />
Advances in Process<br />
Moni<strong>to</strong>ring <strong>and</strong> Control in<br />
Downstream Processing<br />
Networking Refreshment Break Sponsored by Luminex in Exhibit <strong>and</strong> Poster Hall<br />
Point Counterpoint Session:<br />
Integrating Raw Materials<br />
<strong>and</strong> S<strong>up</strong>pliers in<strong>to</strong> a<br />
Pharmaceutical Quality<br />
System (session continues)<br />
Continuous Disposable<br />
Multi-Column<br />
Chroma<strong>to</strong>graphy: Emerging<br />
Technology <strong>to</strong> Enable<br />
More Efficient Downstream<br />
Processing - Sponsored by<br />
Tarpon Biosystems<br />
Cell Culture &<br />
Upstream Processing<br />
Integrating In-Line Process<br />
Moni<strong>to</strong>ring <strong>and</strong> Control<br />
Technologies in Upstream<br />
Processing<br />
12:00 pm Technology Workshops Sponsored by: Diosynth Biotechnology, GE Healthcare, Natrix Separations Inc., <strong>and</strong> New Brunswick Scientific<br />
12:30 pm Networking Luncheon in Exhibit <strong>and</strong> Poster Hall with Dedicated Poster Viewing<br />
Afternoon<br />
Plenary Session – Critical Industry Issues<br />
Networking Refreshment Break Sponsored by Luminex in Exhibit <strong>and</strong> Poster Hall<br />
Keynote Presentations: I. Sustainable Commercial Cell Culture Operations<br />
II. Finding a Home for Process <strong>and</strong> Product Development<br />
5:30 pm Networking Reception in Exhibit <strong>and</strong> Poster Hall Sponsored by BD Biosciences<br />
Thursday, September 23, 2010 <br />
Recovery & Purification<br />
Cell Culture & Upstream<br />
Processing<br />
Emerging Analytical<br />
Requirements<br />
7:15 am Technology Workshop with Continental Breakfast Sponsored by: BD Biosciences<br />
Morning<br />
Plenary Session - Integration of Upstream <strong>and</strong> Downstream Processing<br />
Breakthroughs <strong>to</strong> De-Bottleneck<br />
Downstream Processing<br />
Accelerating & Optimizing Cell Line &<br />
Process Development<br />
Emerging Analytical Requirements <strong>and</strong><br />
their Impact on Process Development<br />
<strong>and</strong> Manufacturing<br />
Networking Refreshment Break in Exhibit <strong>and</strong> Poster Hall<br />
Emerging Analytical Requirements <strong>and</strong><br />
their Impact on Process Development<br />
<strong>and</strong> Manufacturing (session continues)<br />
Immunotherapeutics,<br />
Manufacturing Flexibility <strong>and</strong><br />
Reducing COGS<br />
Lessons Learned from the<br />
2009 Flu Season <strong>to</strong> Guide<br />
Rapid Vaccine<br />
Development <strong>and</strong><br />
Manufacturing Scale Up<br />
Process Development<br />
for Novel Vaccines <strong>and</strong><br />
Immunotherapeutics<br />
Defending Biosimilar<br />
Competition: Bioprocess<br />
IP Protections for Next<br />
Generation Vaccines <strong>and</strong><br />
Immunotherapeutics<br />
Process Development <strong>and</strong><br />
Analytical Characterization<br />
for Vaccine Production<br />
Process Development <strong>and</strong><br />
Analytical Characterization<br />
for Vaccine Production<br />
(session continues)<br />
Strategic Discussion Forum<br />
Smart Flexibility: What Creates the Right<br />
Degree of Flexibility <strong>and</strong> Cost Reduction<br />
in Different Phases of Manufacturing<br />
12:00 pm Technology Workshops Sponsored by: 3M Purification Inc., Irvine Scientific, Applied Biosystems, a part of <strong>Life</strong> Technologies, <strong>and</strong> Invitrogen, a part of <strong>Life</strong> Technologies<br />
12:30 pm Networking Luncheon in Exhibit <strong>and</strong> Poster Hall with Dedicated Poster Viewing<br />
Formulation Strategies for<br />
Protein Therapeutics<br />
Pre-Conference Workshop:<br />
The Formula<strong>to</strong>r of the Future: Using<br />
High Throughput Technologies,<br />
Informatics <strong>and</strong> Rational Design <strong>to</strong><br />
Accelerate <strong>and</strong> Optimize Formulation<br />
Development<br />
Keynote Presentations: Holistic QbD;<br />
Particles, Particles Everywhere<br />
Development of Formulation <strong>and</strong> Drug<br />
Product Design Space<br />
Comparability <strong>and</strong> Characterization<br />
Exercises during Formulation Development<br />
Comparability <strong>and</strong> Characterization<br />
Exercises during Formulation<br />
Development (session continues)<br />
Technology Workshop<br />
Formulation<br />
Strategies for Protein<br />
Therapeutics<br />
Keynote Presentation:<br />
Modeling Protein<br />
Degradation Processes <strong>and</strong><br />
the Development of Rational<br />
Approaches <strong>to</strong> Stabilization<br />
Keynote Presentation:<br />
NIST Perspective on St<strong>and</strong>ards<br />
<strong>and</strong> GMP Processes for<br />
Subvisible Particles in Protein<br />
Therapeutics<br />
Implementing Analytical<br />
Methods <strong>and</strong> Control Steps<br />
for Subvisible Particles<br />
Technology Workshop<br />
Evaluation <strong>and</strong> Control of<br />
Biopharmaceutical Stability<br />
Evaluation <strong>and</strong> Control of<br />
Biopharmaceutical Stability<br />
(session continues)<br />
Formulation Strategies for<br />
Protein Therapeutics<br />
Formulation Strategies for Vaccines<br />
Formulation Impacts of Device <strong>and</strong><br />
Packaging Systems<br />
1:00 pm Featured Presentation in Exhibit <strong>and</strong> Poster Hall: The Enduring Need for Operational Excellence: Lessons Learned from the Oil Field <strong>to</strong> Biotech <strong>and</strong> How Great Companies can Still Fall Short<br />
Implementing the Latest Tools <strong>and</strong><br />
Techniques <strong>to</strong> Optimize the Harvest Step<br />
Host Cell Engineering <strong>to</strong> Improve the<br />
Yield <strong>and</strong> Quality of Biotherapeutics<br />
Development <strong>and</strong> Manufacturing<br />
Strategies for Biosimilars Products<br />
Formulation Development for Next<br />
Generation Biologics<br />
Afternoon<br />
Overcoming Challenges of Production,<br />
Purification <strong>and</strong> Characterization<br />
of Next Generation Antibody-Like<br />
Molecules & Protein Therapeutics<br />
Friday, September 24, 2010 <br />
Networking Refreshment Break in Exhibit <strong>and</strong> Poster Hall (Last chance <strong>to</strong> consult with s<strong>up</strong>pliers <strong>and</strong> view posters)<br />
Implementation of Novel Media<br />
Development <strong>and</strong> Feed Strategies<br />
Site Tour <strong>to</strong> Amgen’s BioNext Facility<br />
(space is limited)<br />
3:00 pm Conference Ends<br />
Recovery & Purification<br />
Cell Culture & Upstream Processing<br />
Evaluation <strong>and</strong> Implementation of Next Generation Purification Technologies<br />
What Comes Next after Titer Increase<br />
Morning<br />
Networking Refreshment Break<br />
Process Characterization for Developing Design Space<br />
Featured Presentation: An Industrial View of Biopharmaceutical Comparability <strong>and</strong> Characterization<br />
Approaches <strong>to</strong> Improve Product Quality <strong>and</strong> Achieve Process Optimization<br />
12:15 pm Technology Workshops Sponsored by: Novozymes Biopharma, Thermo Scientific NanoDrop Products, Rentschler Biotechnologie GmbH<br />
12:45 pm Lunch on your own<br />
Afternoon<br />
Utilizing Continuous Processing <strong>to</strong> Decrease Operation Time <strong>and</strong> Improve Facility Utilization<br />
Applications of Au<strong>to</strong>mated, High-Throughput Technologies in Downstream Processing<br />
Networking Refreshment Break<br />
Advantages of Using Mixed Mode Technologies<br />
Overcoming Challenges of Producing Specific Proteins<br />
Visit www.<strong>IBC</strong><strong>Life</strong><strong>Sciences</strong>.com/BPI for <strong>up</strong>-<strong>to</strong>-date information on this event 4