Goodsell Lesson Plans for MYP Chemistry Moles & Stoichiometry ...
Goodsell Lesson Plans for MYP Chemistry Moles & Stoichiometry ...
Goodsell Lesson Plans for MYP Chemistry Moles & Stoichiometry ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
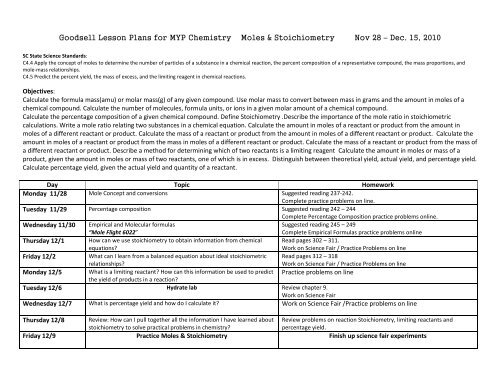
<strong>Goodsell</strong> <strong>Lesson</strong> <strong>Plans</strong> <strong>for</strong> <strong>MYP</strong> <strong>Chemistry</strong> <strong>Moles</strong> & <strong>Stoichiometry</strong> Nov 28 – Dec. 15, 2010<br />
SC State Science Standards:<br />
C4.4 Apply the concept of moles to determine the number of particles of a substance in a chemical reaction, the percent composition of a representative compound, the mass proportions, and<br />
mole-mass relationships.<br />
C4.5 Predict the percent yield, the mass of excess, and the limiting reagent in chemical reactions.<br />
Objectives:<br />
Calculate the <strong>for</strong>mula mass(amu) or molar mass(g) of any given compound. Use molar mass to convert between mass in grams and the amount in moles of a<br />
chemical compound. Calculate the number of molecules, <strong>for</strong>mula units, or ions in a given molar amount of a chemical compound.<br />
Calculate the percentage composition of a given chemical compound. Define <strong>Stoichiometry</strong> .Describe the importance of the mole ratio in stoichiometric<br />
calculations. Write a mole ratio relating two substances in a chemical equation. Calculate the amount in moles of a reactant or product from the amount in<br />
moles of a different reactant or product. Calculate the mass of a reactant or product from the amount in moles of a different reactant or product. Calculate the<br />
amount in moles of a reactant or product from the mass in moles of a different reactant or product. Calculate the mass of a reactant or product from the mass of<br />
a different reactant or product. Describe a method <strong>for</strong> determining which of two reactants is a limiting reagent Calculate the amount in moles or mass of a<br />
product, given the amount in moles or mass of two reactants, one of which is in excess. Distinguish between theoretical yield, actual yield, and percentage yield.<br />
Calculate percentage yield, given the actual yield and quantity of a reactant.<br />
Day Topic Homework<br />
Monday 11/28 Mole Concept and conversions Suggested reading 237-242.<br />
Complete practice problems on line.<br />
Tuesday 11/29 Percentage composition Suggested reading 242 – 244<br />
Complete Percentage Composition practice problems online.<br />
Wednesday 11/30 Empirical and Molecular <strong>for</strong>mulas<br />
“Mole Flight 6022”<br />
Suggested reading 245 – 249<br />
Complete Empirical Formulas practice problems online<br />
Thursday 12/1 How can we use stoichiometry to obtain in<strong>for</strong>mation from chemical<br />
equations<br />
Read pages 302 – 311.<br />
Work on Science Fair / Practice Problems on line<br />
Friday 12/2<br />
What can I learn from a balanced equation about ideal stoichiometric<br />
relationships<br />
Read pages 312 – 318<br />
Work on Science Fair / Practice Problems on line<br />
Monday 12/5 What is a limiting reactant How can this in<strong>for</strong>mation be used to predict Practice problems on line<br />
the yield of products in a reaction<br />
Tuesday 12/6 Hydrate lab Review chapter 9.<br />
Work on Science Fair<br />
Wednesday 12/7 What is percentage yield and how do I calculate it Work on Science Fair /Practice problems on line<br />
Thursday 12/8 Review: How can I pull together all the in<strong>for</strong>mation I have learned about Review problems on reaction <strong>Stoichiometry</strong>, limiting reactants and<br />
stoichiometry to solve practical problems in chemistry<br />
percentage yield.<br />
Friday 12/9 Practice <strong>Moles</strong> & <strong>Stoichiometry</strong> Finish up science fair experiments
Monday 12/12<br />
Science Fair Touch Up<br />
(Meet in Library)<br />
Tuesday 12/13 Practice Unit Test Study some more<br />
Wednesday 12/ 14 Unit Test – <strong>Moles</strong> & <strong>Stoichiometry</strong> Science Fair project due tomorrow<br />
Thursday 12/15 (1/2<br />
Day)<br />
Turn in Science Fair Project<br />
Fun with <strong>Moles</strong><br />
Happy Holidays!