Radial Distribution Functions for the Hydrogen Atom
Radial Distribution Functions for the Hydrogen Atom
Radial Distribution Functions for the Hydrogen Atom
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chemistry 362<br />
Spring 2013<br />
Dr. Jean M. Standard<br />
March 29, 2013<br />
<strong>Radial</strong> <strong>Distribution</strong> <strong>Functions</strong> <strong>for</strong> <strong>the</strong> <strong>Hydrogen</strong> <strong>Atom</strong><br />
The <strong>Radial</strong> <strong>Distribution</strong> Function (RDF) gives <strong>the</strong> probability density <strong>for</strong> finding <strong>the</strong> electron at a radius r from <strong>the</strong><br />
nucleus. The RDF is defined as<br />
RDF = r 2 [ R n ( r)<br />
] 2 .<br />
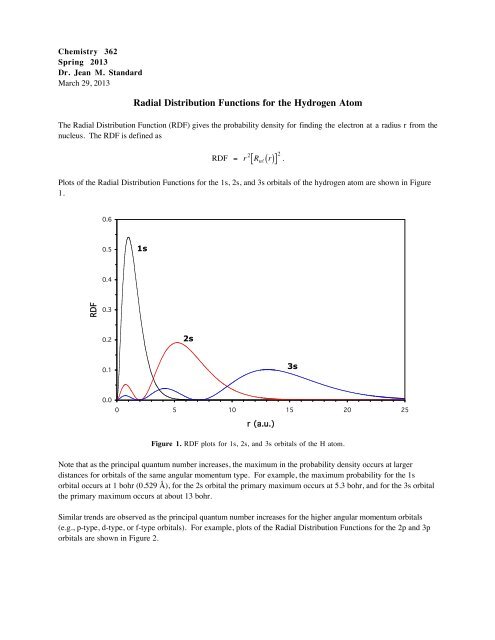
Plots of <strong>the</strong> <strong>Radial</strong> <strong>Distribution</strong> <strong>Functions</strong> <strong>for</strong> <strong>the</strong> 1s, 2s, and 3s orbitals of <strong>the</strong> hydrogen atom are shown in Figure<br />
1.<br />
€<br />
0.6<br />
0.5<br />
1s<br />
0.4<br />
RDF<br />
0.3<br />
0.2<br />
2s<br />
0.1<br />
3s<br />
0.0<br />
0 5 10 15 20 25<br />
r (a.u.)<br />
Figure 1. RDF plots <strong>for</strong> 1s, 2s, and 3s orbitals of <strong>the</strong> H atom.<br />
Note that as <strong>the</strong> principal quantum number increases, <strong>the</strong> maximum in <strong>the</strong> probability density occurs at larger<br />
distances <strong>for</strong> orbitals of <strong>the</strong> same angular momentum type. For example, <strong>the</strong> maximum probability <strong>for</strong> <strong>the</strong> 1s<br />
orbital occurs at 1 bohr (0.529 Å), <strong>for</strong> <strong>the</strong> 2s orbital <strong>the</strong> primary maximum occurs at 5.3 bohr, and <strong>for</strong> <strong>the</strong> 3s orbital<br />
<strong>the</strong> primary maximum occurs at about 13 bohr.<br />
Similar trends are observed as <strong>the</strong> principal quantum number increases <strong>for</strong> <strong>the</strong> higher angular momentum orbitals<br />
(e.g., p-type, d-type, or f-type orbitals). For example, plots of <strong>the</strong> <strong>Radial</strong> <strong>Distribution</strong> <strong>Functions</strong> <strong>for</strong> <strong>the</strong> 2p and 3p<br />
orbitals are shown in Figure 2.
2<br />
0.25<br />
0.20<br />
2p<br />
0.15<br />
RDF<br />
0.10<br />
3p<br />
0.05<br />
0.00<br />
0 5 10 15 20 25<br />
r (a.u.)<br />
Figure 2. RDF plots <strong>for</strong> 2p and 3p orbitals of <strong>the</strong> H atom.<br />
In contrast, if <strong>the</strong> principal quantum number is held fixed and <strong>the</strong> angular momentum type of <strong>the</strong> orbital is varied, we<br />
see from <strong>the</strong> example in Figure 3 that <strong>the</strong> position of <strong>the</strong> primary maximum in <strong>the</strong> probability distribution decreases<br />
as <strong>the</strong> angular momentum increases.<br />
0.15<br />
0.10<br />
3d<br />
3p<br />
3s<br />
RDF<br />
0.05<br />
0.00<br />
0 5 10 15 20 25<br />
r (a.u.)<br />
Figure 3. RDF plots <strong>for</strong> 3s, 3p, and 3d orbitals of <strong>the</strong> H atom.