Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Pioneer</strong> <strong>Junior</strong> <strong>College</strong><br />
H2 Chemistry 9647: Chemical Bonding<br />
Answer to Self-Check Questions<br />
<strong>Pioneer</strong> <strong>Junior</strong> <strong>College</strong><br />
Chemistry Higher 2<br />
Tutorial: Chemical Bonding<br />
q<br />
q<br />
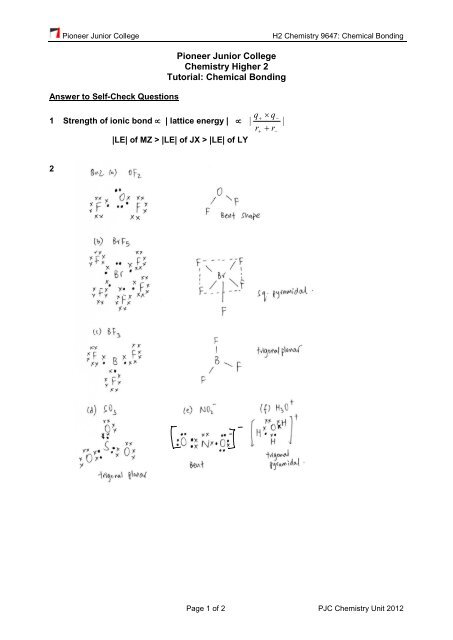
1 Strength of ionic bond | lattice energy | | |<br />
r<br />
r<br />
|LE| of MZ > |LE| of JX > |LE| of LY<br />
2<br />
Page 1 of 2 PJC Chemistry Unit 2012
<strong>Pioneer</strong> <strong>Junior</strong> <strong>College</strong><br />
H2 Chemistry 9647: Chemical Bonding<br />
3 HI, CH 2 Cl 2 , CHF 3<br />
4<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
According to VSEPR, order of repulsion is given by: Lone pair – lone pair<br />
electrons > Lone pair – bond pair electrons > Bond pair – bond pair electrons<br />
CH 4 has no lone pair and 4 bond pairs of electrons hence it has the biggest bond<br />
angle.<br />
NH 3 has 1 lone pair and 3 bond pairs of electrons hence the central atom N<br />
experience greater repulsion.<br />
The 3 bond pair electrons in NH 3 are forced closer together and resulted in a<br />
smaller bond angle than CH 4 .<br />
H 2 O has 2 lone pairs and 2 bond pairs of electrons hence it experiences the<br />
greatest repulsion.<br />
The 2 bond pair electrons in H 2 O are forced even closer than the 3 bond pair<br />
electrons in NH 3 .<br />
Therefore H 2 O has smallest bond angle among the 3 molecules.<br />
Page 2 of 2 PJC Chemistry Unit 2012