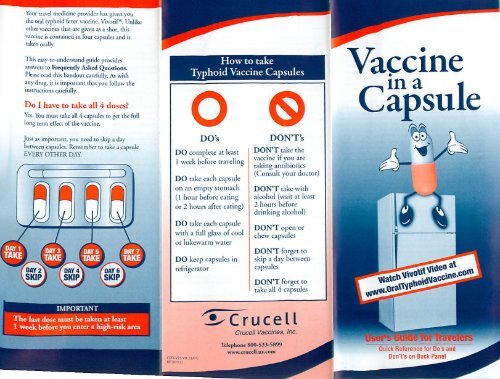
Vaccine in a Capsule: User's Guide for Travelers
Vaccine in a Capsule: User's Guide for Travelers
Vaccine in a Capsule: User's Guide for Travelers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
(<br />
Your travel medic<strong>in</strong>e provider has given you<br />
the oral typhoid fever vacc<strong>in</strong>e, Vivotif°. Unlike<br />
other vacc<strong>in</strong>es that are given as a shot, this<br />
vacc<strong>in</strong>e is conta<strong>in</strong>ed <strong>in</strong> four capsules and is<br />
taken orally.<br />
This easy-to-understand guide provides<br />
answers to Frequently Asked Questions.<br />
Please read this handout carefully. As with<br />
any drug, it is important that you follow the<br />
<strong>in</strong>structions carefully.<br />
Do I have to take all 4 doses<br />
Yes. You must rake all 4 capsules to ger the full<br />
long term effect of the vacc<strong>in</strong>e.<br />
Just as important, you need to skip a day<br />
between capsules. Remember to take a capsule<br />
EVERY OTHER DAY.<br />
(<br />
0<br />
DO's<br />
DO complete at least<br />
1 week be<strong>for</strong>e travel<strong>in</strong>g<br />
DO take each capsule<br />
on an empty stomach<br />
( 3 hour be<strong>for</strong>e eat<strong>in</strong>g<br />
or 2 hours after eat<strong>in</strong>g)<br />
DON'T's<br />
DON'T take the<br />
vacc<strong>in</strong>e if you are<br />
tak<strong>in</strong>g antibiotics<br />
(Consult your doctor)<br />
DON'T take with<br />
alcohol (wait at least<br />
2 hours be<strong>for</strong>e<br />
dr<strong>in</strong>k<strong>in</strong>g alcohol)<br />
<strong>Vacc<strong>in</strong>e</strong><br />
<strong>Capsule</strong><br />
DO take each capsule<br />
with a full glass of cool<br />
or lukewarm water<br />
DO keep capsules <strong>in</strong><br />
refrigerator<br />
DON'T open or<br />
chew capsules<br />
DON'T <strong>for</strong>get to<br />
skip a day between<br />
capsules<br />
DON'T <strong>for</strong>get to<br />
take all 4 capsules<br />
e last dose must be taken at least<br />
1 week be<strong>for</strong>e you enter a high-risk area<br />
Crucell <strong>Vacc<strong>in</strong>e</strong>s, Inc.<br />
V2A VEC (I el.1.7)<br />
Telephone 800-533-5899<br />
www.crucell.us .corn<br />
Quick Reference <strong>for</strong> Do's and<br />
Don't's on Back Panel
v,<br />
How does the oral typhoid<br />
fever vacc<strong>in</strong>e work<br />
This oral vacc<strong>in</strong>e is conta<strong>in</strong>ed <strong>in</strong> a<br />
special type of capsule. This capsule<br />
is designed to dissolve only when<br />
it reaches the small <strong>in</strong>test<strong>in</strong>e. Once<br />
there, the vacc<strong>in</strong>e is absorbed <strong>in</strong>to the<br />
body to beg<strong>in</strong> provid<strong>in</strong>g protection.<br />
Can I dr<strong>in</strong>k alcohol<br />
After tak<strong>in</strong>g a capsule, wait at least TWO<br />
hours be<strong>for</strong>e tak<strong>in</strong>g a dr<strong>in</strong>k of an alcoholic<br />
beverage. The alcohol can cause the capsule to<br />
dissolve be<strong>for</strong>e reach<strong>in</strong>g the small <strong>in</strong>test<strong>in</strong>es_<br />
Also, alcohol itself can destroy the vacc<strong>in</strong>e.<br />
Does each dose have to be taken<br />
at the same time of day<br />
Tak<strong>in</strong>g the vacc<strong>in</strong>e at approximately the same<br />
time will make it easier <strong>for</strong> you to remember.<br />
Many patients f<strong>in</strong>d it easiest to take the capsule<br />
as soon as they wake up <strong>in</strong> the morn<strong>in</strong>g.<br />
Is there an easy way to help<br />
rem<strong>in</strong>d me of when to take<br />
each dose<br />
A sticker is available to help you<br />
remember when to take each of the<br />
4 capsules. Write the day you are<br />
supposed to take each of them <strong>in</strong> the<br />
space provided and put it somewhere<br />
you will see it each day. Your travel<br />
medic<strong>in</strong>e provider may also have<br />
other helpful materials <strong>for</strong> you.<br />
Keep the vacc<strong>in</strong>e<br />
capsules <strong>in</strong> the refrigerator<br />
Precautions<br />
Do not take the oral vacc<strong>in</strong>e if you:<br />
■<br />
Arc tak<strong>in</strong>g antibiotics<br />
• Have had any bad reactions to this oral typhoid<br />
vacc<strong>in</strong>e or emetic coated capsules <strong>in</strong> the past<br />
• Have a fever<br />
• Have cont<strong>in</strong>ued vomit<strong>in</strong>g<br />
■ Have diarrhea or a stomach illness.<br />
Be sure to tell your travel medic<strong>in</strong>e provider if you are<br />
pregnant, nurs<strong>in</strong>g or immunodeficient.<br />
You should still avoid potentially contam<strong>in</strong>ated food or<br />
water, as not all people will be fully protected aga<strong>in</strong>st<br />
typhoid fever.<br />
If you have any additional questions, please discuss them<br />
with your travel medic<strong>in</strong>e provider.<br />
•<br />
Do I have to take the capsule<br />
with water<br />
Yes. It is best to swallow each capsule with a full<br />
glass of cool or room temperature WATER. Don't<br />
open or chew the capsule — remember that the<br />
vacc<strong>in</strong>e capsule needs to reach the small <strong>in</strong>test<strong>in</strong>e<br />
be<strong>for</strong>e it dissolves.<br />
... Can I take each capsule with food<br />
No. The oral vacc<strong>in</strong>e must be taken on an empty<br />
stomach. That means you should take the capsule<br />
either 1 hour be<strong>for</strong>e eat<strong>in</strong>g a meal or 2 hours after<br />
eat<strong>in</strong>g a meal.<br />
Why is it important to take on<br />
an empty stomach<br />
If there is any food <strong>in</strong> the stomach, the capsule<br />
will rema<strong>in</strong> <strong>in</strong> the stomach <strong>for</strong> a<br />
much longer time where the stomach<br />
acids can dissolve the capsule. Once<br />
the capsule is destroyed, the vacc<strong>in</strong>e<br />
will also be destroyed be<strong>for</strong>e it can be<br />
absorbed <strong>in</strong>to the <strong>in</strong>test<strong>in</strong>e. One<br />
hour after tak<strong>in</strong>g the capsule, you<br />
can eat some food.<br />
Side Effects<br />
In cl<strong>in</strong>ical trials, the most frequently reported side<br />
effects were abdom<strong>in</strong>al pa<strong>in</strong>, nausea, headache,<br />
fever, diarrhea, vomit<strong>in</strong>g and sk<strong>in</strong> rash. Although<br />
<strong>in</strong>frequent and mild, nausea was the most mentioned<br />
side effect. You should report any serious side effect<br />
to your doctor or directly to the <strong>Vacc<strong>in</strong>e</strong> Adverse<br />
Event Report<strong>in</strong>g System (1-800-822-7967).<br />
Can a child take the vacc<strong>in</strong>e<br />
Yes, children 6 years and older can take the oral<br />
typhoid vacc<strong>in</strong>e.<br />
vivotift is a regissered trademark uF<br />
Typhoid '<strong>Vacc<strong>in</strong>e</strong> Live Oral Ty2 1 a<br />
Please refer to full product <strong>in</strong><strong>for</strong>mation<br />
on reverse side.
Vivotif®<br />
Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty2la<br />
Description Vivotif® Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty2 la is a liv e attenuated vacc<strong>in</strong>e<br />
<strong>for</strong> oral adm<strong>in</strong>istration only. The vacc<strong>in</strong>e conta<strong>in</strong>s the attenuated stra<strong>in</strong> Salmonella typhi<br />
Ty21a (1.2).<br />
Vivotif® is manufactur ed by the 'Irma Biotech Ltd. The vacc<strong>in</strong>e stra<strong>in</strong> is grown<br />
<strong>in</strong> fermentors under controlled conditions <strong>in</strong> medium conta<strong>in</strong><strong>in</strong>g a digest of yeast<br />
extract. an acid digest of case<strong>in</strong>, dextrose and galactose. The bacteria arc collected by<br />
centrifugation. mixed with a stabilizer conta<strong>in</strong><strong>in</strong>g sucrose, ascorbic acid and am<strong>in</strong>o<br />
acids, and lyophilized. The lyophilized bacteria are mixed with lactose and magnesium<br />
swat= and tilled <strong>in</strong>to gelat<strong>in</strong> capsules which are coated with an organic solution to<br />
render them resistant to dissolution <strong>in</strong> stomach acid. The enteric-coated, salmon/white<br />
capsules are then packaged <strong>in</strong> 4-capsule blisters <strong>for</strong> distribution. The contents of each<br />
enteric-coated capsule arc shown <strong>in</strong> Table I.<br />
Viable S. ryphiTy21a<br />
Non-viable S. ophiTy21a<br />
Sucrose<br />
Ascorbic acid<br />
Am<strong>in</strong>o acid mixture<br />
Lactose<br />
Magnesium mar=<br />
Table It Contents alone enteric-coated capsule<br />
of Vivot<strong>in</strong> (Typhoid <strong>Vacc<strong>in</strong>e</strong> the Oral Ty212)<br />
2-6.8 x 10' colony-<strong>for</strong>m<strong>in</strong>g units'<br />
5-50 x 10' bacterial cells<br />
"6-130 mg<br />
1-5 mg<br />
1.4-7 mg<br />
100-180 mg<br />
3.6-4.4 mg<br />
•Kur<strong>in</strong>e pommy (viable cell counts per capsule) is determ<strong>in</strong>ed by <strong>in</strong>oculation ofagar<br />
plates with appropriate dilutions of the vacc<strong>in</strong>e impended <strong>in</strong> physiological sal<strong>in</strong>e.<br />
Cl<strong>in</strong>ical Pharmacology<br />
Salmonella typhi is the etiological agent of typhoid fever, an acute, febrile enteric<br />
disease. Typhoid fever cont<strong>in</strong>ues to be an important disease <strong>in</strong> many parts of the world.<br />
<strong>Travelers</strong> enter<strong>in</strong>g <strong>in</strong>fected areas are at risk of contract<strong>in</strong>g typhoid fever follow<strong>in</strong>g the<br />
<strong>in</strong>gestion of contam<strong>in</strong>ated food or water. Typhoid fever is considered to be endemic <strong>in</strong><br />
most areas of Central and South America. the African cont<strong>in</strong>ent, the Near East and the<br />
Middle East, Southeast Asia and the Indian subcont<strong>in</strong>ent (3). There are approximately<br />
500 cases of typhoid fever per year diagnosed <strong>in</strong> the United States (4). In 62% of these<br />
patients (data from 1975-1984) the disease was acquired outside of the United States<br />
while <strong>in</strong> 38% of the patients the disease was acquired with<strong>in</strong> the United States (5). Of<br />
340 cases acquired <strong>in</strong> the United States between 1977 and 1979, 239is of the cases were<br />
associated with typhoid carriers. 24% were due to food outbreaks, 23% were associated<br />
with the <strong>in</strong>gestion of contam<strong>in</strong>ated food or water, 6% due to household contact with an<br />
<strong>in</strong>fected person and 4% follow<strong>in</strong>g exposure to S. ryishi <strong>in</strong> a laboratory sett<strong>in</strong>g (6).<br />
The majority of typhoid cases respond favorably to antibiotic therapy. However.<br />
the emergence of multi-drug resistant stra<strong>in</strong>s has greatly complicated therapy and cases<br />
of typhoid fever that ate treated with <strong>in</strong>effective drugs can be fatal (7). Approximately<br />
2-4% of acute typhoid cases result <strong>in</strong> the development of a chronic carrier state (8).<br />
These non-symptomatic carriers are the natural reservoir <strong>for</strong> S. typhi and can serve to<br />
ma<strong>in</strong>ta<strong>in</strong> the disease <strong>in</strong> its endemic state or to directly <strong>in</strong>fect <strong>in</strong>dividuals (3).<br />
Virulent stra<strong>in</strong>s of S. qphi upon <strong>in</strong>gestion are able to pass through the stomach acid<br />
barrier, colonize the <strong>in</strong>test<strong>in</strong>al tract, penetrate the lumen and enter the lymphatic system<br />
and blood stream, thereby caus<strong>in</strong>g disease. One possible mechanism by which disease<br />
may be prevented is by evok<strong>in</strong>g a local immune response <strong>in</strong> the <strong>in</strong>test<strong>in</strong>al tract. Such<br />
local immunity may be <strong>in</strong>duced by oral <strong>in</strong>gestion of a live attenuated stra<strong>in</strong> of S. ryphi<br />
undergo<strong>in</strong>g an aborted <strong>in</strong>fection. The ability of S. typhi to cause disease and to <strong>in</strong>duce<br />
a protective immune response is dependent upon the bacteria possess<strong>in</strong>g a complete<br />
lipopolysaccharide (I). The S. ryphiTy2la vacc<strong>in</strong>e stra<strong>in</strong>. by virtue of a reduction<br />
<strong>in</strong> enzymes essential <strong>for</strong> lipopolysaccharide biosynthesis. is restricted <strong>in</strong> its ability to<br />
„„.,,h, compl ete lipopolysaccharide (1.2). However, a sufficient quantity of complete<br />
phenotype due to the <strong>in</strong>tracellular build-up of <strong>in</strong>termediates dur<strong>in</strong>g lipopolysaccharide<br />
synthesis (1,2).<br />
Results from cl<strong>in</strong>ical studies <strong>in</strong>dicate that adults and children greater than 6<br />
years of age may be protected aga<strong>in</strong>st typhoid fever follow<strong>in</strong>g the oral <strong>in</strong>gestion of<br />
4 doses of Vivotif® (Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty21a). The efficacy of the S. ryphi<br />
1Y2la stra<strong>in</strong> has been evaluated <strong>in</strong> a series of randomized, double-bl<strong>in</strong>d, controlled<br />
field trials. Suspected typhoid cases, detected by passive surveillance, were confirmed<br />
bacteriologically either by blood or bone marrow culture. The first trial was per<strong>for</strong>med<br />
<strong>in</strong> Alexandria, Egypt with a study population of 32,388 children aged 6 to 7 years.<br />
Three doses of vacc<strong>in</strong>e, <strong>in</strong> the <strong>for</strong>m of a freshly reconstituted suspension adm<strong>in</strong>istered<br />
after <strong>in</strong>gestion of 1 g of bicarbonate. were given on alternate days. Immunization<br />
resulted <strong>in</strong> a 95% decrease [95% confidence <strong>in</strong>terval (CI) = 77%-99%j <strong>in</strong> the<br />
<strong>in</strong>cidence of typhoid fever over a 3-year period of surveillance (9). A series of field<br />
trials were subsequently per<strong>for</strong>med <strong>in</strong> Santiago. Chile to evaluate efficacy when the<br />
vacc<strong>in</strong>e stra<strong>in</strong> was adm<strong>in</strong>istered <strong>in</strong> the <strong>for</strong>m of an acid-resistant enteric-coated capsule.<br />
The <strong>in</strong>itial trial <strong>in</strong>volved 82.543 school-aged children, and compared 1 or 2 doses of<br />
vacc<strong>in</strong>e given one week apart. After 24 months of surveillance vacc<strong>in</strong>e efficacy was 29%<br />
(95% Cl = 4%-47%) <strong>for</strong> the s<strong>in</strong>gle dose schedule and 59% (95% CI = 41(14-71%)<br />
<strong>for</strong> the 2-dose schedule (10). A further field trial was per<strong>for</strong>med <strong>in</strong> Santiago, Chile<br />
<strong>in</strong>volv<strong>in</strong>g 109,594 school-aged children (I I). Three doses of enteric-coated capsules<br />
were adm<strong>in</strong>istered either on alternate days (short immunization schedule) or 21 days<br />
apart (long immunization schedule). Follow<strong>in</strong>g 36 months of surveillance vacc<strong>in</strong>ation<br />
resulted <strong>in</strong> a 67% (95% CI = 47%-79%) decrease <strong>in</strong> the <strong>in</strong>cidence of typhoid fever <strong>in</strong><br />
the short immunization schedule group and a 49% reduction (95% CI = 24%-66%)<br />
<strong>in</strong> the long immunization schedule group. After 48 months of surveillance the short<br />
immunization schedule resulted <strong>in</strong> a 69% (95% CI = 55%-80%) decrease its typhoid<br />
fever (12). An undim<strong>in</strong>ished level of protection was observed dur<strong>in</strong>g the fifth year<br />
of surveillance. A field trial was next conducted <strong>in</strong> Santiago, Chile to determ<strong>in</strong>e the<br />
relative efficacy of 2. 3 and 4 doses of enteric-coated vacc<strong>in</strong>e adm<strong>in</strong>istered on alternate<br />
days to school-aged children. Relative vacc<strong>in</strong>e efficacy as determ<strong>in</strong>ed by comparison<br />
of disease <strong>in</strong>cidence with<strong>in</strong> the three vacc<strong>in</strong>ated groups was highest <strong>for</strong> the four dose<br />
regimen (13). The <strong>in</strong>cidence of typhoid fever per 105 study subjects was 160.5 (95%<br />
CI = 130-191) <strong>for</strong> the three dose regimen versus 95.8 (95% CI = 71-121) <strong>for</strong> the four<br />
dose regimen (p
Vivotif®<br />
Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty2la<br />
In<strong>for</strong>mation .fill Pntienu<br />
It is essential that all 4 doses of vacc<strong>in</strong>e be taken at the prescribed alternate day<br />
<strong>in</strong>terval to obta<strong>in</strong> a maximal protective immune response. <strong>Vacc<strong>in</strong>e</strong> potency is dependent<br />
upon storage under refrigeration (between 2 °C and 8 "C (35.6"F- 46.4 °HI. "Ile<br />
vacc<strong>in</strong>e should be stored under refrigeration at all times. It is essential to replace unused<br />
vacc<strong>in</strong>e <strong>in</strong> the refrigerator between doses. The vacc<strong>in</strong>e capsule should be swallowed<br />
approximately 1 hour be<strong>for</strong>e a meal with a cold or hike-warm (temperature not to<br />
exceed body temperature. e.g.. 37 °C (98.6 °HI dr<strong>in</strong>k. Care should be taken not to<br />
chew the vacc<strong>in</strong>e capsule. The vacc<strong>in</strong>e capsule should be swallowed as soon after plac<strong>in</strong>g<br />
<strong>in</strong> the mouth as possible.<br />
Not all recipients of Vivotif® (Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty21 a) will be fully<br />
protected aga<strong>in</strong>st typhoid fever. <strong>Travelers</strong> should take all necessary precautions to<br />
avoid contact or <strong>in</strong>gestion of potentially contam<strong>in</strong>ated food or water. Several antimalaria<br />
drugs. such as mefloqu<strong>in</strong>e, chloroqu<strong>in</strong>e and proguanil (not approved <strong>for</strong> use<br />
<strong>in</strong> US) possess antibacterial activity which may <strong>in</strong>terfere with the immunogenicity of<br />
Vivotif®. Cl<strong>in</strong>ical results (see Warn<strong>in</strong>gs - Drug-Interactions) <strong>in</strong>dicate that mefloqu<strong>in</strong>e<br />
and chloroqu<strong>in</strong>e can be adm<strong>in</strong>istered together with Vivotif®. Proguanil should be<br />
adm<strong>in</strong>istered only if 10 days or more have elapsed s<strong>in</strong>ce the f<strong>in</strong>al dose of Vivotif® was<br />
<strong>in</strong>gested. Any serious adverse reactions related to the adm<strong>in</strong>istration of the vaccitse<br />
should be reported to your health care provider. You may also report an adverse<br />
reaction directly to the <strong>Vacc<strong>in</strong>e</strong> Adverse Event Report<strong>in</strong>g System (1-800-822-7967)<br />
(20). Your health care provider should <strong>in</strong><strong>for</strong>m you of the benefits and risks of the<br />
vacc<strong>in</strong>e, the importance of tak<strong>in</strong>g all 4 capsules <strong>in</strong> the correct schedule, and the<br />
importance of proper storage temperature of the capsules.<br />
Carr<strong>in</strong>osertesit, Mutagenetis, Impairment of Fertility<br />
Long-term studies <strong>in</strong> animals with Vivotif® have not been per<strong>for</strong>med to evaluate<br />
carc<strong>in</strong>ogenic potential, mutagenic potential or impairment of fertility.<br />
Pregnanty Category C<br />
Animal reproduction studies have not been conducted with Vivotiki). It is not<br />
known whether Vivotif® can cause fetalharm when adm<strong>in</strong>istered to pregnant women<br />
or can affect reproduction capacity. Vivotif® should be given to a pregnant woman only<br />
if clearly needed.<br />
Nurs<strong>in</strong>g Mothers There is no data to warrant the use of this product <strong>in</strong> nurs<strong>in</strong>g<br />
mothers. It is not known if Vivotif® is excreted <strong>in</strong> human milk.<br />
Pediatric Use The safety and efficacy of Vivotif® has not been established <strong>in</strong> children<br />
under 6 years of age. This product is not <strong>in</strong>dicated <strong>for</strong> use <strong>in</strong> children under 6 years<br />
of age.<br />
Adverse Reactions<br />
More than 1.4 million doses of Ty21a have been adm<strong>in</strong>istered <strong>in</strong> controlled cl<strong>in</strong>ical<br />
trials and more than 150 million doses of Vivotif® (Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty21a)<br />
have been marketed world-wide. Active surveillance <strong>for</strong> adverse reactions of entericcoated<br />
capsules was per<strong>for</strong>med <strong>in</strong> a pilot study (21) and <strong>in</strong> a subgroup of a large field<br />
trial (14) <strong>in</strong>volv<strong>in</strong>g a total of 483 <strong>in</strong>dividuals receiv<strong>in</strong>g three vacc<strong>in</strong>e doses. The overall<br />
symptom rates from both studies when vacc<strong>in</strong>ated with capsules were comb<strong>in</strong>ed and<br />
shown to be: abdom<strong>in</strong>al pa<strong>in</strong> (6.4%). nausea (5.8%), headache (4.8%), fever (3.3%),<br />
diarrhea (2.9%), vomit<strong>in</strong>g (1.5%) and sk<strong>in</strong> rash (1.0%). Only the <strong>in</strong>cidence of nausea<br />
occured at a statistically higher frequency <strong>in</strong> the vacc<strong>in</strong>ated group as compared to the<br />
placebo group (14). Adm<strong>in</strong>istration of vacc<strong>in</strong>e doses more than 5-fold higher titan the<br />
currently recommended dose caused only mild reactions <strong>in</strong> an open study <strong>in</strong>volv<strong>in</strong>g 155<br />
healthy adult males (16).<br />
Post-market<strong>in</strong>g surveillance has revealed that adverse reactions are <strong>in</strong>frequent and<br />
mild (17). Adverse reactions reported to the manufacturer dur<strong>in</strong>g 1991-1995, dur<strong>in</strong>g<br />
which time over 60 million doses (eilpstde‘) were adm<strong>in</strong>istered, <strong>in</strong>cluded: diarrhea<br />
(N = 45), abdom<strong>in</strong>al pa<strong>in</strong> (N = 42). nausea (N 35), fever (N = 34), headache<br />
(N = 26). sk<strong>in</strong> rash (N = 26). vomit<strong>in</strong>g (N = 18). or urticaria <strong>in</strong> the trunk and/or<br />
extremities (N 13). O ne isolated, non-fatal anaphylactic shock considered to he an<br />
allergic reaction to the vacc<strong>in</strong>e was reported.<br />
Dosage and Adm<strong>in</strong>istration<br />
One capsule is us be swallower! approximately 1 hour be<strong>for</strong>e a meal with a cold or<br />
lake-warns (temperature not to exceed hotly temperature. e.g.. 37 °C (98.6 °H) dr<strong>in</strong>k<br />
on alternate days, e.g., days 1, 3. 5 and 7. Immunization (<strong>in</strong>gestion of all 4 doses of<br />
Vivotif® (Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty21 a) should he completed at least I week prior<br />
to potential exposure to S. ophi.<br />
The blister conta<strong>in</strong><strong>in</strong>g the vacc<strong>in</strong>e capsules should be <strong>in</strong>spected to ensure that the<br />
Mil seal and capsules are <strong>in</strong>tact. The vacc<strong>in</strong>e capsule should not be chewed and should<br />
be swallowed as soon after plac<strong>in</strong>g <strong>in</strong> the mouth as possible. A complete immunization<br />
schedule is the <strong>in</strong>gestion of 4 vacc<strong>in</strong>e capsules as described above.<br />
Re-immunization<br />
The optimum booster schedule <strong>for</strong> Vivotif® has not been determ<strong>in</strong>ed. Efficacy has<br />
been shown to persist <strong>for</strong> at least 5 years. Further, there is no experience with Vivotif®<br />
as a booster <strong>in</strong> persons previously immunized with parenteral typhoid vacc<strong>in</strong>e. It is<br />
recommended that a re-immunization dose consist<strong>in</strong>g of four vacc<strong>in</strong>e capsules taken<br />
on alternate days be given every 5 years under conditions of repeated or cont<strong>in</strong>ued<br />
exposure to typhoid fever (7).<br />
How Supplied<br />
A s<strong>in</strong>gle foil blister conta<strong>in</strong>s 4 doses of vacc<strong>in</strong>e <strong>in</strong> a s<strong>in</strong>gle package.<br />
Storage<br />
Vivotif® (Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Tv21a) is not stable when exposed to ambient<br />
temperatures. Vivotif® should there<strong>for</strong>e he shipped and stored between 2 °C and 8 °C.<br />
(35.6 °F-46.4 F.ach package of vacc<strong>in</strong>e shows an expiration date. This expiration<br />
date is valid only if the product has been ma<strong>in</strong>ta<strong>in</strong>ed at 2 °C-8 (35.6"F-46.4 °H.<br />
Manufactured by<br />
Berna Biotech Ltd, Berne. Switzerland US-Licence No. 1632<br />
Distributed by<br />
Berna Products, Coral Gables. H. 33146 Ric only<br />
References<br />
1. Germanier R.. E. hirer. Isolation and characterisation of Gal E mutant Ty21a of<br />
Salmonella dyphi: a candidate stra<strong>in</strong> <strong>for</strong> a live, oral typhoid vacc<strong>in</strong>e. J. Infect. Dis.<br />
131: 553-558. 1975.<br />
2. Germanier R.. E. Hirer. Characteristics of the attenuated oral vacc<strong>in</strong>e stra<strong>in</strong> S. typhi<br />
Ty21a. Develop. Biol. Standard 53: 3-7. 1983.<br />
3. Miller 5.1.. E.L. Hohmann. D.A. Pegues. Salmonella (<strong>in</strong>clud<strong>in</strong>g Salmonelb typ<strong>in</strong>).<br />
In: Pr<strong>in</strong>ciples and practice of <strong>in</strong>fectious diseases. G.L. Mandell. J.E. Bennett, R.<br />
Dol<strong>in</strong> (cd.) fourth edition, Churchill Liv<strong>in</strong>gstone Inc. 2013-2033. 1995.<br />
4. Centers <strong>for</strong> Disease Control. Summary of notifiable diseases United States 1995.<br />
MMWR 44 (Supplement), 1996.<br />
5. Ryan C.A.. N.T. Hargrett-Bean, P.A. Blake. Salmonella typhi <strong>in</strong>fections <strong>in</strong> the<br />
United SLOGS. 1975-1984: Increas<strong>in</strong>g role of <strong>for</strong>eign travel. Rev. Infect. Dis. 11:<br />
1-8. 1989.<br />
6. Taylor D.N.. R.A. Pollard, EA. Blake. Typhoid <strong>in</strong> the United States and the Risk to<br />
the International Traveler. J. Infect. Dis. 148: 599-602, 1983.<br />
7. Recommendations of the Advisory Committee on Immunization Practices (ACID):<br />
Typhoid Immunization. NINIWR 43 (R11-14). 1994.<br />
8. Allies W.R., M. Robb<strong>in</strong>s. Age and sex as factors <strong>in</strong> the development of the typhoid<br />
carrier state, and a model <strong>for</strong> estimat<strong>in</strong>g carrier prevalence. Am. J. Public !kalif'<br />
33: 221-230, 1943.<br />
9. Wandan N1.11., C. Serie, Y. Cerisier, S. Sallam, R. Germanier. A controlled field<br />
trial of live Salmonella w,ln stra<strong>in</strong> Ty21a oral vacc<strong>in</strong>e aga<strong>in</strong>st typhoid: three-year<br />
results. J. Infect. Div. 145: 292-296, 1982.<br />
10. Black R.E.. M.M. Lev<strong>in</strong>e. C. Ferreccio. M.l_ Clentents, C. Lanata, J. Rooney, R.<br />
Germanier, Chilean Typhoid Committee. Efficacy done or two doses of Ty2la<br />
S/ ‘<br />
<strong>Vacc<strong>in</strong>e</strong> 8,8<br />
e/2 Lryipẖ8i4. e2C1ci9n0e.<strong>in</strong> 9 enteric-coated capsules <strong>in</strong> a controlled field trial.<br />
11. Lev<strong>in</strong>e M.M.. C. Ferreccio, R.E. Black, R. Germanier. Chilean Typhoid<br />
Committee. Large-Scale Field Trial of Ty21a Typhoid <strong>Vacc<strong>in</strong>e</strong> Live Oral Ty21a <strong>in</strong><br />
Enteric-Coated <strong>Capsule</strong> Formulation. Lancet I: 1049-1052. 1987.<br />
12. Lev<strong>in</strong>e M.M., C. Ferocccio, R.E. Black. CO. 'racket, R. Germanier , Chilean<br />
Typhoid Committee. Progress <strong>in</strong> vacc<strong>in</strong>es aga<strong>in</strong>st typhoid fever. Rev. Infect. Dis. 11<br />
(Supplement 3): S552-5567, 1989.<br />
13. Ferreccio C.,N13.1. Lev<strong>in</strong>e. H. Rodriguez. R. Contreras. Chilean Typhoid<br />
Committee. Comparative efficacy of two, three. or four doses of Ty2 la live oral<br />
typhoid vacc<strong>in</strong>e <strong>in</strong> enteric-coated capsules: a field trial <strong>in</strong> endemic area. J. Infect.<br />
Dis. 159: 766-769. 1989.<br />
14. Simanjuntak C.H.. F.P. Paicologo. N.H. Punjabi, R. Darmowigoto, Soeprawoloi<br />
II. Totosudirjo, P Haryanto. E. Suprijanto, N.D. Witham. S.L. Hoffman. Oral<br />
immunisation io 9 typhoid fever <strong>in</strong> Indonesia with Ty2Ia vacc<strong>in</strong>e. Lancet 338:<br />
15. Data on File. Swiss Serum and <strong>Vacc<strong>in</strong>e</strong> Institute Berne, Switzerland.<br />
16. Gilman R.H., R.B. Hornick, W.E. Woodward, H.L. DuPont. M.J. Snyder, M.M.<br />
Lev<strong>in</strong>e. J.P. Libonati. Evaluation of a UDP-glucose-4-epimeraseless mutant of<br />
Salmonella ophi as a live oral vacc<strong>in</strong>e. J. Infect. Dis. 136: 717-723. 1977.<br />
17. Cryz. S.J. Jr., Post•market<strong>in</strong>g experience with live oralTy21a <strong>Vacc<strong>in</strong>e</strong>. Lancet: 341:<br />
49-50, 1993. Data on File, Swiss Serum and <strong>Vacc<strong>in</strong>e</strong> Institute Berne. Switzerland.<br />
18. llotowitz H., CA. Carlxmaro, Inhibition of the Salmonella oephi oral vacc<strong>in</strong>e stra<strong>in</strong><br />
Ty21a, by mefloqu<strong>in</strong>e and chloroqu<strong>in</strong>e. J. Infect. Dis. 166: 1462-1464, 1992.<br />
19. Kollatitsch H., J.U. Que, C. Kunz, G. Wiedermann, C. Herzog, S.J. Cryz Jr. Safety<br />
and immunogenicity of live oral cholera and typhoid vacc<strong>in</strong>es adm<strong>in</strong>istered alone<br />
20. (1)r 9<br />
with anti-malarial drugs, oral polio vacc<strong>in</strong>e or yellow fever<br />
vacc<strong>in</strong>e. J. Infect. Dis. 175: 871-875, 1997.<br />
39: 730-73.3.<br />
<strong>Vacc<strong>in</strong>e</strong> Adverse Event Report<strong>in</strong>g System - United States. MMWR<br />
1990.<br />
21. Lev<strong>in</strong>e M.M., R.E. Black. C. Ferreccio, M.L. Clements, C. Lanata• J.<br />
Rooney.<br />
.<br />
e iaami ru<strong>in</strong>t:d i c.r .i1nTh 1 c<br />
ontrol led yfield (e )i lst. Idsnt:onD ct omp:1:9ni. fpd a i nwe s i an ji.d9H8D60.riumgs greas:1A. nst<br />
bCon ference . Stockholm, m<br />
L<strong>in</strong>dberg and R. Mollby<br />
Lund. 9Sweden,<br />
Version: August 2°06