Polytropic Process of an Ideal Gas
Polytropic Process of an Ideal Gas
Polytropic Process of an Ideal Gas
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
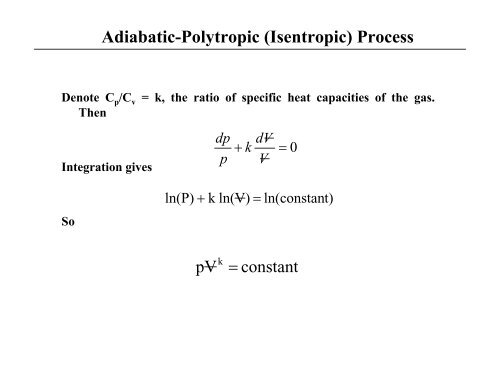
Adiabatic-<strong>Polytropic</strong> (Isentropic) <strong>Process</strong><br />
Denote C p /C v = k, the ratio <strong>of</strong> specific heat capacities <strong>of</strong> the gas.<br />
Then<br />
Integration gives<br />
dp<br />
p<br />
+<br />
k<br />
dV<br />
V<br />
= 0<br />
So<br />
ln(P) + k ln(V) =<br />
ln(const<strong>an</strong>t)<br />
pV<br />
k =<br />
const<strong>an</strong>t