You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
In the Lab<br />
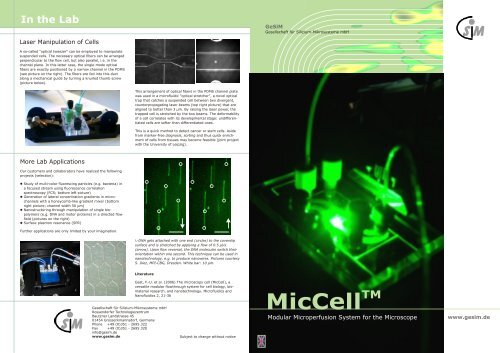
Laser Manipulation of Cells<br />
A so-called "optical tweezer" can be employed to manipulate<br />
suspended cells. The necessary optical fibers can be arranged<br />
perpendicular to the flow cell, but also parallel, i.e. in the<br />
channel plane. In this latter case, the single-mode optical<br />
fibers are exactly positioned by a narrow channel in the PDMS<br />
(see picture on the right). The fibers are fed into this duct<br />
along a mechanical guide by turning a knurled thumb screw<br />
(picture below).<br />
More Lab Applications<br />
Our customers and collaborators have realized the following<br />
projects (selection):<br />
� Study of multi-color fluorescing particles (e.g. bacteria) in<br />
a focused stream using fluorescence correlation<br />
spectroscopy (FCS; bottom left picture)<br />
� Generation of lateral concentration gradients in microchannels<br />
with a honeycomb-like gradient mixer (bottom<br />
right picture; channel width 50 µm)<br />
� Nanostructuring through manipulation of single biopolymers<br />
(e.g. DNA and motor proteins) in a directed flow<br />
field (pictures on the right)<br />
� Surface plasmon resonance (SPR)<br />
Further applications are only limited by your imagination.<br />
This arrangement of optical fibers in the PDMS channel plate<br />
was used in a microfluidic "optical stretcher", a novel optical<br />
trap that catches a suspended cell between two divergent,<br />
counterpropagating laser beams (top right picture) that are<br />
aligned to better than 3 µm. By raising the laser power, the<br />
trapped cell is stretched by the two beams. The deformability<br />
of a cell correlates with its developmental stage: undifferentiated<br />
cells are softer than differentiated ones.<br />
This is a quick method to detect cancer or stem cells. Aside<br />
from marker-free diagnosis, sorting and thus quick enrichment<br />
of cells from tissues may become feasible (joint project<br />
with the University of Leipzig).<br />
�-DNA gets attached with one end (circles) to the coverslip<br />
surface and is stretched by applying a flow of 0.5 µl/s<br />
(arrow). Upon flow reversal, the DNA molecules switch their<br />
orientation within one second. This technique can be used in<br />
nanotechnology, e.g. to produce nanowires. Pictures courtesy<br />
S. Diez, MPI-CBG, Dresden. White bar: 10 µm.<br />
Literature<br />
Gast, F.-U. et al. (2006) The microscopy cell (<strong>MicCell</strong>), a<br />
versatile modular flowthrough system for cell biology, biomaterial<br />
research, and nanotechnology. Microfluidics and<br />
Nanofluidics 2, 21-36<br />
Gesellschaft für Silizium-Mikrosysteme <strong>mbH</strong><br />
Rossendorfer Technologiezentrum<br />
Bautzner Landstrasse 45<br />
01454 Grosserkmannsdorf, Germany<br />
Phone +49 (0)351 - 2695 322<br />
Fax +49 (0)351 - 2695 320<br />
info@gesim.de<br />
www.gesim.de Subject to change without notice<br />
<strong>GeSiM</strong><br />
Gesellschaft für Silizium-Mikrosysteme <strong>mbH</strong><br />
<strong>MicCell</strong><br />
<strong>TM</strong><br />
Modular Microperfusion System for the Microscope<br />
www.gesim.de
<strong>TM</strong><br />
The <strong>MicCell</strong><br />
Possible Applications<br />
The study of biomolecules or cells often includes their immobilization<br />
on surfaces. Microfluidic flowthrough systems are only<br />
suited if the channels can be opened and closed reversibly, if<br />
possible without tinkering with adhesive tape. Highly desirable<br />
would be a modular fluidic system that can be easily adapted to<br />
diverse analytical methods (e.g. electrochemical or optical) and<br />
also efficiently cleaned.<br />
For this purpose, <strong>GeSiM</strong> has developed microperfusion chambers<br />
with removable coverslip for the microscope. By using a novel,<br />
patented setup, these flow cells are mounted in seconds and can<br />
be applied in cell imaging or single-molecule fluorescence experiments.<br />
If needed, electrical signals can be processed or optical<br />
fibers mounted. The system is completed by a computer-controlled<br />
macrofluidic environment.<br />
Layout and assembly of the system can be modified in a wide<br />
range. So a bunch of different materials can be used and microvalves<br />
and microelectrodes can be integrated. Due to the easy<br />
and modular setup, even the most complicated method in<br />
screening, diagnostics, and nanotechnology can be realized in a<br />
customer-specific way.<br />
Semi-automatic drug screening using adherent cells or tissue slices in<br />
the microscope<br />
Interaction of cells with immobilized proteins, oligosaccharides, lipids,<br />
and other ligands<br />
Viability testing (e.g. for cancer cells) and other cell-based tests<br />
Cell adhesion tests with prepared surfaces<br />
Laser manipulation of cells in the flow: e.g. with the "optical stretcher"<br />
(for cancer diagnosis and to isolate stem cells)<br />
DNA transfection in the flow<br />
Measurement of the homogeneity of microbeads and size sorting<br />
Single-molecule detection unsing fluorescent biomolecules (fluorescence<br />
correlation spectroscopy in the flow, receptor-ligand binding studies,<br />
motor protein kinetics, hybridization kinetics on microarrays, etc.)<br />
Capillary electrophoresis (CE)<br />
Application of hydrodynamic flow fields (e.g. to align macromolecules,<br />
nanotechnology)<br />
Generation of lateral concentration gradients in a microchannel<br />
Activity measurment of electrically active cells on microelectrode arrays<br />
Rapid prototyping of microsystems (e.g. for "stopped flow" or for<br />
chemical synthesis) by use of PDMS<br />
Study of non-transparent substrates in the flow with the fully revolvable<br />
"sample carrier", etc.<br />
Microelectrodes<br />
Microelectrodes for heating and sensing are microstructured<br />
in our cleanroom and those regions that must not come in<br />
contact with the medium are passivated by vapor deposition<br />
of SiO .<br />
2<br />
In the assembled <strong>MicCell</strong>, electrode pads are contacted by<br />
spring contacts and the signals are then conducted to a<br />
socket via a printed circuit board (shown in green). If<br />
coverslip and channel plate are from glass, electrodes can be<br />
present at both the top and the bottom of the channels.<br />
Examples shown were<br />
performed in cooperation<br />
with Fraunhofer-IBMT,<br />
Berlin, and Max-Planck<br />
Institute for Polymer<br />
Research, Mainz<br />
Sample Carrier for Opaque Objects<br />
The <strong>MicCell</strong> channel is normally translucent and thus allows<br />
sample illumination from the rear side. Opaque objects can<br />
be introduced into the channel with a pivotable sample carrier<br />
(orange in the schematic drawing below) and observed in the<br />
flow, e.g. for material testing.<br />
<strong>GeSiM</strong> offers such a flowthrough cell that can hold objects up<br />
to a size of 2.5x2.5 mm. As sample and sample holder are<br />
intransparent, the sample must be illuminated from the<br />
objective side, i.e. along the optical path. Its specialty: the<br />
specimen can be rotated a full 360° around the optical axis of<br />
the microscope, such that objects can be exposed to the flow<br />
at an arbitrary angle.<br />
Optional Design<br />
Microelectrode Arrays (MEAs)<br />
To measure signals of muscle or nerve cells, these cells can<br />
be grown on coverslips containing microelectrode arrays (see<br />
bottom picture) that are then mounted into a flow channel.<br />
The flow system shown can process electrical potentials while<br />
the cells are being inspected under a microscope.<br />
Potential (mV)<br />
1.0<br />
0.5<br />
0.0<br />
-0.5<br />
0 50 100 150 200<br />
t (msec)
Accessories<br />
Hydrogel Microvalve<br />
To add liquids to the main channel, e.g. to start and stop<br />
reactions, a dead volume-free microvalve must be placed in<br />
the flow channel. The patented <strong>GeSiM</strong> hydrogel valve contains<br />
hydrogel particles that dramatically swell by hydration<br />
when the temperature drops below 34 °C, hence blocking the<br />
channel. Warming above this transition temperature opens<br />
the valve immediately, which allows the aspiration of<br />
substances into the main channel.<br />
Several versions of this valve exist (see separate brochure),<br />
but in the <strong>MicCell</strong> only those are used where the fluid passes<br />
vertically through holes etched into the heated actuator<br />
chamber. This very small device can be built into a standard<br />
UNF 1/4-28 fitting that can be screwed into a <strong>MicCell</strong> inlet<br />
hole.<br />
Actuator Specifications<br />
Scanning EM picture<br />
of an opened actuator<br />
chamber with<br />
dehydrated hydrogel<br />
particles<br />
� Hydrogel polymerized from purified N-isopropylacrylamide<br />
(PNIPAAm)<br />
� Standard transition temperature 34 °C (can be adjusted),<br />
more than tenfold volume increase upon cooling ("normally<br />
closed valve")<br />
� Heating power max. 250 mW at 3.5 - 5 V, heating and<br />
temperature measurement via sputtered platinum electrodes<br />
� Switching period ca. 1 - 3 seconds; if Peltier cooling is<br />
used, closing about as fast<br />
5<br />
� Watertight up to a pressure of ~ 6·10 Pa (6 bar)<br />
� Needs aqueous solutions, but tolerates<br />
< 15 % methanol, ethanol, acetone<br />
< 5 % 1-propanol<br />
Hydrogel valve PV7 without<br />
printed circuit board (thus<br />
autoclavable) in the <strong>MicCell</strong><br />
Sample injection into T and K-channel. The inlet and outlet of<br />
the main channel are marked green. After blocking the main<br />
inlet, samples can be sucked into the channel via the red<br />
hydrogel valve. The K-channel (right panel) has an extra<br />
outlet (white circle) to flush the dead volume between microvalve<br />
and T-junction.<br />
Microvalve filled with hydrogel particles, before (left) and<br />
after (right) packaging<br />
Hydrogel valve<br />
Electrical<br />
connection<br />
Standard fitting<br />
(to microstructure)<br />
Hydrogel particle actuator with small dead volume (PV6) in<br />
standard UNF fitting. Left: injector for manual sample addition<br />
via a microfunnel, right: automatic sample injection<br />
through capillary (inner diameter 25 - 762 µm).<br />
Hydrodynamic flow chamber with eight hydrogel valves<br />
(arrows) to generate flow in various directions (in collaboration<br />
with the University of Technology Dresden and Namos<br />
G<strong>mbH</strong>, Dresden)<br />
Assembly<br />
The <strong>MicCell</strong> microsystem consists of<br />
� the channel plate from PDMS or glass that contains the<br />
microstructured channel system<br />
� the coverslip<br />
The microchannel is covered by a coverslip that is firmly<br />
pressed onto the channel plate as it is mounted between the<br />
lid (polycarbonate, on the top) and support (metal, on the<br />
bottom). Feeding and draining of the fluid is achieved by<br />
openings in the channel plate.<br />
The easy fluidic connection of the microchannel via standard<br />
fittings in the <strong>MicCell</strong> lid is groundbreaking. The flow is generated<br />
by external syringe pumps ("macrofluidics") that are<br />
much more reliable than integrated microfluidic pumps.<br />
A light microscope is not included in the shipment.<br />
Components<br />
� Working plate: metal adapter to hold the <strong>MicCell</strong> support in<br />
practically all inverted and many upright and stereomicroscopes<br />
(required)<br />
� Fluid processor: computer-controlled flow control box containing<br />
one or more syringe pumps, multi-port distribution<br />
valve, 2/2-way valve, and controller for hydrogel valve<br />
(recommended)<br />
� Tubing with small inner diameter (0.3 mm, 0.5 mm etc.),<br />
UNF fittings (1/4-28), O-rings<br />
� Hydrogel microvalves (see below)<br />
� Windows control software (see below) for interactive operation<br />
and process automation with the fluid processor<br />
(flow ramps, valve control, temperature control)<br />
� Optional integrated devices: micromixer, thin film heater<br />
and thermosensor, calorimetric flow sensor (<strong>GeSiM</strong> specialty,<br />
see extra brochure), pressure sensor, microelectrode<br />
array, impedance sensor, "sample carrier"<br />
<strong>MicCell</strong> Control Software<br />
For rinsing, precincuation, and the timely starting and stopping<br />
of reactions, all devices (syringe pumps, valves), plus<br />
the velocity and direction of the flow, can be interactively<br />
controlled via a Windows software. In addition, complex<br />
procedures from several single functions (pumps and valves,<br />
delay time, repeats) can be automated using an easy<br />
scripting language (example in the bottom right figure).<br />
Of course, price-concious researchers can use their own fluidic<br />
periphery, but will then lose all the comfort of a graphic<br />
user interface and programmable operation.<br />
Principle<br />
Zuleitungen<br />
Channel plate<br />
with microchannel<br />
Coverslip<br />
Metal support<br />
<strong>MicCell</strong> assembly. One can see a channel plate of glass with a<br />
polymer channel structured on it; normally it consists of<br />
molded PDMS. Fastening elements and the working plate<br />
(adapter to the microscope) are not shown.<br />
Control software for two syringe<br />
pumps, 6/1 distribution valve ,<br />
and hydrogel valve. On the right:<br />
programming example<br />
Lid<br />
<strong>MicCell</strong> in an inverted<br />
microscope with fluid<br />
processor (on the left),<br />
controlled by a notebook.<br />
Standard fluid processor<br />
with syringe pump (left<br />
side), hydrogel valve<br />
controller and 2/2 valve<br />
(middle), and 4/1 distribution<br />
valve (right). In<br />
front of the fluid processor,<br />
a <strong>MicCell</strong>, complete<br />
with hydrogel valve and<br />
blue metal support, is<br />
mounted in the black<br />
working adapter plate.
PDMS Flow Cell<br />
PDMS Channel Plate<br />
Although the channel plate can be made of various materials<br />
(glass, silicon, ceramics), a good and easy-to-handle material<br />
is PDMS (polydimethylsiloxane), a two-component silicone.<br />
The PDMS mixture is poured onto a master tool of silicon and<br />
cured to form a soft polymer block that seals tightly against<br />
the coverslip.<br />
PDMS molding is a well established lab technique, however it<br />
is often rather bricolage than anything else. <strong>GeSiM</strong> has<br />
developed a professional casting station in which the inlet<br />
and outlet holes are kept open by "channel spacers" (see<br />
picture on the right). The specialty: the lid not only defines<br />
the PDMS thickness during molding, but is also the lid in the<br />
fully assembled flow cell; it presses together coverslip and<br />
microchannel and contains all fluidic connections. So all you<br />
need to do after curing is to replace the master with the<br />
coverslip, screw in the right fittings, and start your experiments.<br />
The channel plate can be used several times, by the<br />
way.<br />
Instead of purchasing the casting station, you can let <strong>GeSiM</strong><br />
manufacture the PDMS channel plate, even hundred units per<br />
month. The lids will be reused.<br />
Due to its easy handling and flexible use, the PDMS channel<br />
plate can be an ideal tool for rapid prototyping: to make a<br />
new channel, only a new master is required.<br />
Casting stations for coverslips of size 22 x 22 and 22 x 50<br />
mm, with metal base plate, silicon master (black), Teflon<br />
case (white), polycarbonate lid (transparent), and channel<br />
spacer (brown)<br />
Advantages of PDMS<br />
� Transparent, with no background fluorescence<br />
� Easy to cast<br />
� Sealing without problems<br />
� Biocompatible (at least after plasma treatment)<br />
� Covalent bonding by plasma treatment<br />
Disadvantages of PDMS<br />
� Faint background in transmitted light<br />
� Permeable for small molecules such as gases (O , CO ),<br />
2 2<br />
alcohols, etc. (actually an advantage in cell biology)<br />
Principle<br />
Inlet<br />
Lid<br />
Channel spacers Master<br />
Silicon molding<br />
master<br />
Casting of the<br />
PDMS channel<br />
plate (top) and<br />
use as flow cell<br />
(bottom)<br />
PDMS<br />
Coverslip<br />
PDMS molding in the<br />
casting station. The<br />
silicon master is under<br />
the transparent lid.<br />
Please note the channel<br />
spacers (brown<br />
fittings).<br />
Demolded PDMS<br />
channel plate with<br />
coverslip. The channel<br />
spacers were replaced<br />
with green fittings.<br />
PDMS channel plate,<br />
mounted with coverslip<br />
on a metal support,<br />
with hydrogel valve<br />
microinjector<br />
Customized Solutions<br />
<strong>GeSiM</strong>'s great specialty is to care for individual needs of<br />
customers. Aside from the numerous possible channel geometries<br />
and materials, the entire setup can also vary in a wide<br />
range. Solutions have been devised for many problems,<br />
among them flowthrough cells that are used without microscope,<br />
e.g. with heating plate for microarray hybrization or to<br />
measure surface plasmon resonance (SPR). Variants for other<br />
microscopes (such as stereomicroscopes) and the integration<br />
of microelectrodes are also possible.<br />
It is therefore a rather rare event that two <strong>MicCell</strong>s are absolutely<br />
identical when they leave the factory.<br />
With polymer walls and microelectrodes...<br />
For SPR...<br />
Flow Cells of Different Size<br />
For channel systems that are larger than a classical coverslip<br />
(22 x 22 mm), up to the size of a slide, the arrangement of<br />
the <strong>MicCell</strong> is somewhat different, as shown in these examples.<br />
Standard PDMS <strong>MicCell</strong>s with coverslips of size 22 x 50 mm<br />
(top) and 22 x 22 mm (bottom)<br />
Variations on a Theme...<br />
With optical fibers...<br />
In a stereomicroscope (see<br />
also below)...<br />
With glass flow cell<br />
and electrodes on<br />
both sides of the<br />
channel...<br />
With glass T-channel and<br />
hydrogel valve injector<br />
(arrow)...<br />
To heat slides...<br />
Coverslip 50<br />
x 22 mm on<br />
a polymer<br />
channel<br />
structured on<br />
a slide.<br />
Sealing is<br />
achieved by<br />
a layer of<br />
silicone<br />
rubber that<br />
was screenprinted<br />
onto<br />
the channel<br />
walls.<br />
Large PDMS<br />
channel plate<br />
used in a<br />
stereomicroscope.<br />
The<br />
channel is<br />
covered by a<br />
slide.