Pre-Lab Preparation Sheet
Pre-Lab Preparation Sheet
Pre-Lab Preparation Sheet
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Experiment 2<br />
CH 3<br />
H H CH 3<br />
OH<br />
OH<br />
How Can We Tell the<br />
Difference Between<br />
Stereoisomers and Why<br />
is it Important for<br />
NSAID Drugs<br />
CH 3<br />
O<br />
S-Naproxen<br />
CH 3<br />
S-Ibuprofen<br />
O<br />
CH 3<br />
O<br />
H<br />
O<br />
H<br />
OH<br />
OH<br />
CH 3<br />
O<br />
R-Naproxen<br />
H CH 3<br />
O<br />
R-Ibuprofen<br />
O<br />
H<br />
O<br />
OH<br />
CH 3<br />
OH<br />
O<br />
O<br />
S-Ketoprofen R-Ketoprofen<br />
Introduction:<br />
Most of us have taken Advil, Midol IB, Motrin, Nuprin, Actron, Orudis, Aleve, Anaprox or<br />
Naprosyn as a pain releaver at some time or other. These drugs are a sub group of the Nonsteroidial<br />
Anti-inflammatory class of drugs (NSAIDS) that also inculdes aspirin, celebrex, vioxx<br />
and bextra among others. NSAIDS have a range of medicinal effects: analgesic (pain relief) antiinflammatory<br />
(reduction of inflammation in wounds and joints) and antipyretic (reduction of<br />
fever). They are often recommended or prescribed for chronic problems such as arthritis. More<br />
information on NSAIDS is available from the following website and its links<br />
(http://www.medicinenet.com/nonsteroidal_antiinflammatory_drugs/article.htm). There has<br />
been considerable controversy as to the safety of some NSAIDS, with two drugs vioxx and<br />
betrex having been removed form the market in recent years.<br />
NSAID produce their effects by inhibiting the class of enzymes called cyclooxygenases, which<br />
catalyze the formation of prostaglandins and thromboxane from arachidonic acid. One such<br />
reaction is provided in Equation 1. There is a large variety of prostaglandins that have various<br />
physiological effects.<br />
O<br />
O<br />
OH<br />
OH<br />
COX<br />
+ H<br />
+ 2 O 2 O<br />
2<br />
Aracadonic Acid Prostaglandin PGH 2<br />
O<br />
H<br />
OH<br />
O<br />
Equation 1<br />
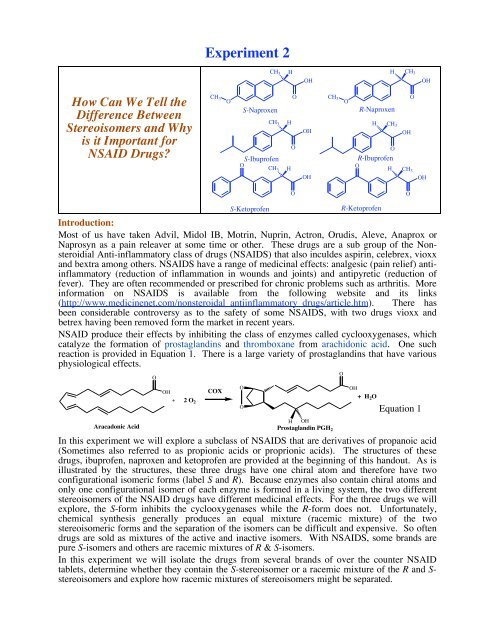
In this experiment we will explore a subclass of NSAIDS that are derivatives of propanoic acid<br />
(Sometimes also referred to as propionic acids or proprionic acids). The structures of these<br />
drugs, ibuprofen, naproxen and ketoprofen are provided at the beginning of this handout. As is<br />
illustrated by the structures, these three drugs have one chiral atom and therefore have two<br />
configurational isomeric forms (label S and R). Because enzymes also contain chiral atoms and<br />
only one configurational isomer of each enzyme is formed in a living system, the two different<br />
stereoisomers of the NSAID drugs have different medicinal effects. For the three drugs we will<br />
explore, the S-form inhibits the cyclooxygenases while the R-form does not. Unfortunately,<br />
chemical synthesis generally produces an equal mixture (racemic mixture) of the two<br />
stereoisomeric forms and the separation of the isomers can be difficult and expensive. So often<br />
drugs are sold as mixtures of the active and inactive isomers. With NSAIDS, some brands are<br />
pure S-isomers and others are racemic mixtures of R & S-isomers.<br />
In this experiment we will isolate the drugs from several brands of over the counter NSAID<br />
tablets, determine whether they contain the S-stereoisomer or a racemic mixture of the R and S-<br />
stereoisomers and explore how racemic mixtures of stereoisomers might be separated.
Experiment 2: NSAIDS 2<br />
Week 1<br />
A. How can we isolate the drugs<br />
<strong>Pre</strong>-lab <strong>Pre</strong>paration Assignment:<br />
1. Read:<br />
-This Experiment handout<br />
and<br />
-Classification of unknown compounds from their solubilities in aqueous media: CHEM<br />
211 <strong>Lab</strong> Manual p. A1.<br />
-Extraction (<strong>Lab</strong> Manual Part V. A pp. A1-A2 and Padías: pp. 116-127)<br />
-Stereoisomers: <strong>Lab</strong> Manual Part V A: sec. A->D. 3. (pp. B1-B6).<br />
-<strong>Pre</strong>pare for Exploration questions on pp. B2-B6.<br />
2. Complete the <strong>Pre</strong>lab activity on the course website by 5:00 PM Sunday, February 1.<br />
3. In your lab notebook: (See <strong>Lab</strong> Manual pp. 14-16 for format.)<br />
a. Set up the Table of Contents<br />
b. Enter the title of this experiment<br />
4. Bring your notebook to the Monday <strong>Lab</strong> Discussion period.<br />
5. After the lab discussion, enter the Waste Disposal instructions for this experiment into your<br />
notebook in the appropriate place.<br />
Question of the Week:<br />
How can we isolate propanoic acid NSAIDS from their tablets<br />
In order to produce tablets that are easily consumed and can survive the shipping and handling<br />
necessary to get them to drug stores, active ingredients, in this case the NSAID molecules, are<br />
mixed with binding materials. Binding materials are largely made up of starch, a mixture of<br />
large neutral (neither acidic nor basic) molecules.<br />
After completing the pre-lab assignment, come to the Monday, lab discussion period with<br />
suggestions as to how to answer the Question of the Week. You will be working with the same<br />
lab group as youi did on Experiment 1.<br />
Key Terms/concepts/techniques:<br />
• Acid-Base Reactions<br />
• Extractions<br />
• Configurational Isomers<br />
• Enantiomers<br />
• Diastereomers<br />
•<br />
•<br />
Epimers<br />
Meso compounds<br />
Safety Matters:<br />
• Safety glasses are required.<br />
• No sandals or open-toe shoes