Clostridium Difficile (C-Diff) testing - West Hertfordshire Hospitals ...
Clostridium Difficile (C-Diff) testing - West Hertfordshire Hospitals ...
Clostridium Difficile (C-Diff) testing - West Hertfordshire Hospitals ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Trust Offices<br />
Watford General Hospital<br />
Vicarage Road<br />
Watford<br />
<strong>Hertfordshire</strong><br />
WD18 0HB<br />
Tel: 01923 436209<br />
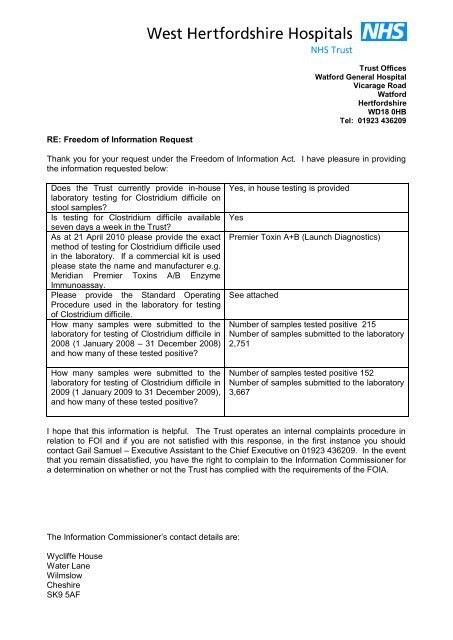
RE: Freedom of Information Request<br />
Thank you for your request under the Freedom of Information Act. I have pleasure in providing<br />
the information requested below:<br />
Does the Trust currently provide in-house<br />
laboratory <strong>testing</strong> for <strong>Clostridium</strong> difficile on<br />
stool samples<br />
Is <strong>testing</strong> for <strong>Clostridium</strong> difficile available<br />
seven days a week in the Trust<br />
As at 21 April 2010 please provide the exact<br />
method of <strong>testing</strong> for <strong>Clostridium</strong> difficile used<br />
in the laboratory. If a commercial kit is used<br />
please state the name and manufacturer e.g.<br />
Meridian Premier Toxins A/B Enzyme<br />
Immunoassay.<br />
Please provide the Standard Operating<br />
Procedure used in the laboratory for <strong>testing</strong><br />
of <strong>Clostridium</strong> difficile.<br />
How many samples were submitted to the<br />
laboratory for <strong>testing</strong> of <strong>Clostridium</strong> difficile in<br />
2008 (1 January 2008 – 31 December 2008)<br />
and how many of these tested positive<br />
How many samples were submitted to the<br />
laboratory for <strong>testing</strong> of <strong>Clostridium</strong> difficile in<br />
2009 (1 January 2009 to 31 December 2009),<br />
and how many of these tested positive<br />
Yes, in house <strong>testing</strong> is provided<br />
Yes<br />
Premier Toxin A+B (Launch Diagnostics)<br />
See attached<br />
Number of samples tested positive 215<br />
Number of samples submitted to the laboratory<br />
2,751<br />
Number of samples tested positive 152<br />
Number of samples submitted to the laboratory<br />
3,667<br />
I hope that this information is helpful. The Trust operates an internal complaints procedure in<br />
relation to FOI and if you are not satisfied with this response, in the first instance you should<br />
contact Gail Samuel – Executive Assistant to the Chief Executive on 01923 436209. In the event<br />
that you remain dissatisfied, you have the right to complain to the Information Commissioner for<br />
a determination on whether or not the Trust has complied with the requirements of the FOIA.<br />
The Information Commissioner’s contact details are:<br />
Wycliffe House<br />
Water Lane<br />
Wilmslow<br />
Cheshire<br />
SK9 5AF
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 1 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
PROCEDURES<br />
TITLE<br />
Document No. (Q-PULSE)<br />
<strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
MI/SOP/040<br />
EDITION No. 2<br />
HOSPITAL SITE<br />
LABORATORY DISCIPLINE<br />
HHGH & WGH<br />
Microbiology<br />
DATE OF ISSUE June 2008<br />
REVIEW DATE June 2010<br />
AUTHOR<br />
REVIEWED BY<br />
AUTHORISED BY<br />
Kathy Weber<br />
Bill Champion/Rachel Hilson<br />
Dr Prema Seetul-Singh<br />
COPY 1<br />
LOCATION OF COPIES<br />
COSHH REFERENCE<br />
UNCONTROLLED IF:<br />
1. Q-Pulse<br />
2. HHGH Microbiology Laboratory<br />
3. WGH Microbiology Laboratory<br />
Body Fluids/Waste, TECHLAB C.DIFFICILE TOX<br />
A/B, Hycolin, hazard class 2 pathogens<br />
Not printed on blue paper<br />
Document review history<br />
Review date Reviewed by Signature<br />
June 2008<br />
Rachel Hilson<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 2 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
1. HEALTH & SAFETY<br />
1. Gloves must be worn when handling potentially infectious stool samples and<br />
whilst performing the test procedure.<br />
2. The kit should be handled as though capable of transmitting infection.<br />
Please refer to the following documents:<br />
The laboratory Health and Safety policy<br />
Relevant COSHH data sheets<br />
Risk assessment for the Procedure<br />
2. PRINCIPLE OF ASSAY<br />
Background<br />
Toxin producing <strong>Clostridium</strong> difficile is a major cause of antibiotic associated diarrhoea<br />
and is an important nosocomial pathogen. It causes a wide spectrum of symptoms,<br />
from asymptomatic carriage to severe diarrhoea and life-threatening<br />
pseudomembraneous colitis.<br />
Laboratory diagnosis is based on detection of this organism and/or either of its two<br />
major toxins, toxins A and B. Toxin A is an enterotoxin, causing increase intestinal<br />
permeability and subsequent fluid accumulation and diarrhoea. Toxin B is cytotoxic for<br />
most mammalian cell lines in vitro. It has been hypothesized that the two toxins have<br />
an additive or synergistic effect in vivo.<br />
Not all strains of <strong>Clostridium</strong> difficile produce toxins and those, which do, can produce<br />
toxin over a wide range of concentrations.<br />
Diagnosis of <strong>Clostridium</strong> difficile associated diarrhoea (CDAD) has generally been<br />
achieved by isolation of <strong>Clostridium</strong> difficile from the stool specimen and detection of<br />
toxin B in the specimen using cell culture with neutralisation of the toxin by specific<br />
antiserum.<br />
Although the assay is very sensitive and has a high correlation to patients with severe<br />
disease, it requires cell culture capability and up to 72 hours of incubation to complete.<br />
This therefore has certain limitations in terms of patient intervention. Also the cytotoxin<br />
assay is not standardised, is subjective in interpretation and detects the actions of toxin<br />
B rather than toxin A.<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 3 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
Principle<br />
<strong>Clostridium</strong> difficile toxin detection at WHHT is performed by Enzyme Immuno Assay<br />
(EIA) using the Premier <strong>Clostridium</strong> <strong><strong>Diff</strong>icile</strong> Toxins A + B kit supplied by Meridian<br />
Diagnostics (1) .<br />
The test utilises mouse monoclonal anti toxin A antibody and polyclonal goat antitoxin<br />
B antibody absorbed to microtitre plates. An enzyme conjugated antitoxin A + B<br />
polyclonal antibody and diluted patient stool samples are incubated in wells of a<br />
microtitre plate. If either toxin is present in the patient sample, an enzyme-antibody<br />
complex is formed which binds to the solid phase forming a sandwich. After washing to<br />
remove unbound conjugate the substrate tetramethylbenzidine (TMB) is added and<br />
incubated. A coloured product develops in the presence of any bound enzyme.<br />
3. RESPONSIBILITY<br />
Test set-up – Trained MLA or BMS staff<br />
Interpretation – BMS staff or Trainee BMS staff under supervision<br />
4. SAMPLE REQUIREMENTS<br />
Stool specimens must be collected into clean, airtight containers with no preservatives.<br />
Prior to <strong>testing</strong>, specimens should be stored at 2-8°C for a maximum of 72 hours.<br />
If <strong>testing</strong> cannot be performed within this time specimens should be frozen immediately<br />
upon receipt and stored at –80C until tested.<br />
Do not use stools that have dried out.<br />
All stool samples must be mixed thoroughly, regardless of consistency, to ensure a<br />
representative sample prior to specimen preparation.<br />
5. EQUIPMENT/REAGENTS/CALIBRATORS<br />
12 x 75 mm plastic disposable test tubes<br />
Wooden applicator sticks<br />
Vortex mixer<br />
Incubator 37 C +-2 C<br />
Timer<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 4 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
Premier <strong>Clostridium</strong> <strong><strong>Diff</strong>icile</strong> Toxins A + B kit supplied by Meridian Diagnostics<br />
Allow all reagents to reach room temperature before use.<br />
Antibody coated Microwells.<br />
(COSHH – Biological)<br />
Coated with mouse monoclonal antitoxin A antibody + polyclonal goat anti toxin B<br />
antibody.<br />
Positive Control<br />
(COSHH – Biological)<br />
Inactivated toxin A + B in buffered protein solution with a preservative.<br />
Sample Diluent / Negative control<br />
Protein solution with a preservative.<br />
(COSHH – Biological)<br />
20X Wash Buffer<br />
(COSHH – Irritant)<br />
Concentrated wash buffer with a preservative.<br />
Prepare 1 x wash buffer as required from 20 x stock concentration provided i.e. 5ml<br />
concentrated wash buffer + 95ml distilled H 2 0. Place in a clean wash bottle. The<br />
working wash buffer should be stored at room temperature for up to three months.<br />
Label clearly with the date of preparation and date of expiry.<br />
Enzyme Conjugate<br />
(COSHH – Harmful)<br />
Polyclonal goat anti-toxin A _ anti-toxin B antibodies conjugated to horseradish<br />
peroxidase in buffered protein solution containing a preservative.<br />
Substrate<br />
(COSHH – Harmful)<br />
Buffered solution containing urea peroxide and tetramethylbenzidine.<br />
Stop Solution<br />
1M phosphoric acid.<br />
(COSHH – Irritant)<br />
Caution : Avoid contact with skin.<br />
6. PROCEDURE/CALIBRATION<br />
Criteria for C.difficile Toxin Testing<br />
1. Only diarrhoeal samples that are runny, like water and conform to the Kings<br />
stool chart (KSC) J, K or L<br />
2. And are from patients > 65 years old (in-patients, out patients or GP’s)<br />
3. If specifically requested and conform to the KSC as J, K or L<br />
The following samples will not be tested:<br />
1. Samples that do not conform to the Kings stool chart J, K, and L.<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 5 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
2. Samples that are from patients under 2 years old.<br />
3. Samples from patients who have tested positive within the previous 28 days.<br />
NB Refer to appendix B: Criteria for <strong>testing</strong> C.difficile<br />
Specimen Preparation<br />
1. Add 200µl of sample diluent to a 12 x 75 mm plastic disposable test tube.<br />
2. Mix stool as thoroughly as possible prior to pipetting.<br />
3. Using a transfer pipette, sample stool to 50µl calibration point (first mark on<br />
pipette) and dispense into the sample diluent. Rinse pipette out in the sample<br />
diluent several times to expel the entire stool sample. Seal tube with Para film<br />
or a cap and vortex for 15 seconds.<br />
Test Procedure<br />
1) Print a WQS worksheet. Using ARR (refer to PA/SOP/IT/101 ARR Microbiology<br />
Medical Abstract Reporting) check for any samples that have been positive within<br />
the last 28 days.<br />
2) Break off the required number of microwells (one well for each specimen plus one<br />
positive and one negative control well per batch). Place the microwells in the<br />
microwell strip holder. Record microwell positions of controls and specimens.<br />
Unused strips must be resealed in the pouch immediately.<br />
3) Using a transfer pipette add 100µl of diluted stool (second calibrated mark on<br />
pipette) to the appropriate well. Place pipette tip halfway into well and allow sample<br />
to run slowly down the side of the well.<br />
4) Add two free falling drops of Positive control and for the negative control add 200µl<br />
sample diluent to a tube, using a transfer pipette add 100µl (second calibrated mark<br />
on pipette) to the appropriate well.<br />
5) Add one free falling drop of enzyme conjugate (50µl) to all wells.<br />
6) Seal wells with a strip of plate sealer and mix by firmly shaking or swirling the plate<br />
(or use a plate shaker) for 30 seconds.<br />
7) Incubate for 50 minutes at 37°C (± 2°C).<br />
8) Carefully remove the plate sealer and wash the wells as follows:<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 6 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
a) Firmly tap the contents into a waste receptacle.<br />
b) Tap the inverted plate on a clean pad of absorbent paper.<br />
c) Fill all wells with 1 x wash buffer directing the stream of buffer to the sides of the<br />
wells to avoid foaming.<br />
d) Repeat this cycle four times. Ensure that the last tap onto absorbent paper<br />
removes as much excess wash buffer as possible, but do not allow the wells to<br />
completely dry out at any time.<br />
e) Wipe the underside of the wells with lint free tissue.<br />
9) Add two drops of substrate to each well.<br />
10) Gently swirl the micro-titre plate (or use a plate shaker) for 30 seconds to mix the<br />
substrates.<br />
11) Incubate for 10 minutes at room temperature.<br />
12) Add two drops of Stop Solution to all wells and again shake the plate for 30<br />
seconds. Allow colour to develop for two minutes before reading. Leave for 15<br />
minutes before reporting negatives.<br />
13) Read the plate either<br />
(a) Visually<br />
(b) Spectrophotometrically using an EIA microwell reader at 450nm<br />
(Reference filler 630nm) within 30 minutes of adding the stop solution.<br />
Visual reading<br />
NEGATIVE control - colourless to faint (barely visible) yellow<br />
POSITIVE control - definite yellow colour<br />
Spectrophotometric reading Using dual wavelength (450/630nm).<br />
NEGATIVE control – OD values should be less than 0.100 but greater than 0.000.<br />
POSITIVE control – OD values should be less than 2.000 but greater than 0.100.<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 7 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
7.QUALITY CONTROL AND ASSESSMENT<br />
The Positive and Negative controls must be used with each batch of specimens to<br />
provide quality assurance of reagents and the results recorded on the work list.<br />
8. LIMITATIONS<br />
Premier Toxins A + B detects the presence of C.difficile toxin A + B in stools.<br />
Failure to detect toxin A or toxin B in stool from patients with suspected<br />
C.difficile associated disease may not preclude actual disease, but may be due<br />
to such factors as improper collection, handling and storage of specimens.<br />
The level of Toxin A or B in the stool has not been shown to correlate with either<br />
the presence or severity of disease. Results should therefore be interpreted<br />
together with other laboratory tests and clinical information.<br />
Specimens should ideally be stored at 2-8°C for less than 24 hours. Prolonged<br />
storage will cause deterioration of Toxin A in the sample, especially so in liquid<br />
stools.<br />
Most stools will remain reactive for up to 3 days if stored at 2-8°C.<br />
9. REPORTING RESULTS<br />
Recording Results<br />
As well as recording the results on the form, also fill in the following information on the<br />
WQS worksheet:<br />
Results<br />
Positive and negative control results<br />
Initials of staff performing and validating the test<br />
Batch number and expiry date of kit and buffer<br />
This should then be photocopied twice and stored in the CDT file on the faeces bench.<br />
Copies given to:<br />
1) ICN<br />
2) Consultant<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 8 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
Negative results are reported on the computer without referral to medical staff using<br />
the Pathnet code: CDT N<br />
Positive results are reported on the computer without referral to medical staff using<br />
the Pathnet code: CDT P<br />
Copies of positive forms are given to:<br />
1) ICN<br />
2) Debbie Dover<br />
Centre For Infections<br />
Once reported, place the request form on the COSERV clip. Refer to current<br />
guidelines on notification and microbiological surveillance. (Refer to MI/RP/001 on<br />
CDSC and COSURV reporting).<br />
Infection Control Staff<br />
Inform the infection control team of all positive results.<br />
Weekly Sheet<br />
All Positives must be entered onto the weekly C. difficile data Table MI/F/011.<br />
At weekends record if all the tests were negative, to enable quick collation of the data<br />
for Trust offices, micro consultants and ICN’s.<br />
10. REFERENCES<br />
1. http://www.mdeur.com/products/616096.htm<br />
2. Advisory Committee on Dangerous Pathogens 2004 Approved List of Biological<br />
Agents. http://www.hse.gov.uk/pubns/misc208.pdf. p. 1-17.<br />
3. Public Health Laboratory Service Standing Advisory Committee on Laboratory<br />
Safety. Safety Precautions: Notes for Guidance. 4th ed. London: Public Health<br />
Laboratory Service (PHLS); 1993.<br />
4. Control of Substances Hazardous to Health Regulations 2002. General COSHH<br />
Approved Code of Practice and Guidance, L5. Suffolk: HSE Books; 2002.<br />
5. Health and Safety Executive. 5 steps to risk assessment: a step-by-step guide to<br />
a safer and Healthier workplace, IND (G) 163 (REVL). Suffolk: HSE Books;<br />
2002.<br />
6. Health and Safety Executive. A guide to risk assessment requirements: common<br />
provisions in health and safety law, IND (G) 218 (L). Suffolk: HSE Books; 2002.<br />
7. Health Services Advisory Committee. Safety in Health Service laboratories. Safe<br />
working and the Prevention of infection in clinical laboratories and similar<br />
facilities. 2nd ed. Suffolk: HSE Books;<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 9 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
Related Documents<br />
MI/LP/013 CDT Testing Notes<br />
MI/LP/016 CDT Test Procedure<br />
MI/F/011 <strong>Clostridium</strong> difficile Data Table<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 10 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
APPENDIX A: MI/F/011<br />
CLOSTRIDIUM DIFFICILE DATA<br />
Week:<br />
Name Sex Date of<br />
birth<br />
Lab<br />
Number<br />
Hospital<br />
number<br />
NHS<br />
Number<br />
Ward /<br />
Location<br />
Hosp<br />
or<br />
GP<br />
Date of<br />
specimen<br />
Date of<br />
test<br />
65 Date entered<br />
on CoSurv<br />
Date entered<br />
on MESS<br />
reporting<br />
These sheets need to be filled in weekly and returned to Debbie Dover. Please write legibly and do not use green ink.<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 11 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
Appendix B Criteria for Testing C.difficile<br />
PATIENT<br />
CDT<br />
requested<br />
Diarrhoeal<br />
sample<br />
(Kings<br />
stool<br />
chart J, K,<br />
L)<br />
Age 265 COE Test Computer code<br />
Inpatient/outpatient/GP / X X X X<br />
Inpatient/outpatient/GP X / X X X X X<br />
CDT R *(not<br />
tested because..)<br />
Inpatient/outpatient/GP X X<br />
Inpatient/outpatient/GP X X X<br />
X<br />
CDT N/ CDT P<br />
CDT NT<br />
Inpatient/outpatient/GP X X X X X X<br />
Inpatient/outpatient/GP X X X X X<br />
Inpatient/outpatient/GP X X CDT N/ CDT P<br />
Inpatient/outpatient/GP X X X X CDT NT<br />
Inpatient/outpatient/GP X X X CDT N/ CDT P<br />
Inpatient/outpatient/GP X X X X X X<br />
Patients who have<br />
tested positive within<br />
the previous 28 days<br />
X CDT PR<br />
* CDT R free text in: The performance of this assay has not been evaluated in a paediatric population + asymptomatic carriers<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 12 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
Authorised users<br />
I have read the above procedure and been trained to use it. I undertake to follow it in all<br />
details<br />
NAME Post Signature Date<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
<strong>West</strong> <strong>Hertfordshire</strong> NHS <strong>Hospitals</strong> Trust<br />
Microbiology Department Page 13 of 13<br />
Title: <strong>Clostridium</strong> difficile Toxin Detection by<br />
E.I.A<br />
Date of Issue June 2008<br />
PROCEDURE AMENDMENT SHEET – FOR MINOR CHANGES ONLY<br />
Number Date Page<br />
No.<br />
1<br />
Amendment<br />
Authorised<br />
by:<br />
2<br />
3<br />
4<br />
5<br />
6<br />
7<br />
8<br />
The amendment must be authorised by the author, where possible.<br />
The amendment must be underlined and an asterisk written in the margin alongside the<br />
change – DO NOT USE LIQUID PAPER<br />
No more than 8 amendments will be made before the procedure is revised.<br />
MAJOR CHANGES MUST RESULT IN AN IMMEDIATE PROCEDURE REVISION<br />
MI/SOP/040 <strong>Clostridium</strong> difficile Toxin Detection by E.I.A<br />
CONTROLLED COPY<br />
NO PHOTOCOPIES OR UNAUTHORISED AMENDMENTS ARE TO BE MADE
Yours sincerely<br />
Jan Filochowski<br />
Chief Executive