Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Mass Spectrometry Appendix D1 Part V. Techniques and Theory<br />
Appendix D.<br />
Mass Spectrometry<br />
Mass Spectra Theory & Practice (See also: Padías pp. 98 - 105 & CGWW pp. 50-56)<br />
In Mass Spectral Analysis, organic molecules are bombarded by high energy electrons.<br />
Impact of an electron on a molecule transfers a large amount of energy to the molecule.<br />
M + * e - M * + e -<br />
One way that the molecule can dissipate the excess energy is to lose an electron and become<br />
a Radical Cation the "Molecular Ion".<br />
M * M + . + e -<br />
The mass spectrometer is designed to separate and count cations according to their<br />
charge/mass ratio (m/z). Since most ions have lost only one electron, m/z = mass of the<br />
ion.<br />
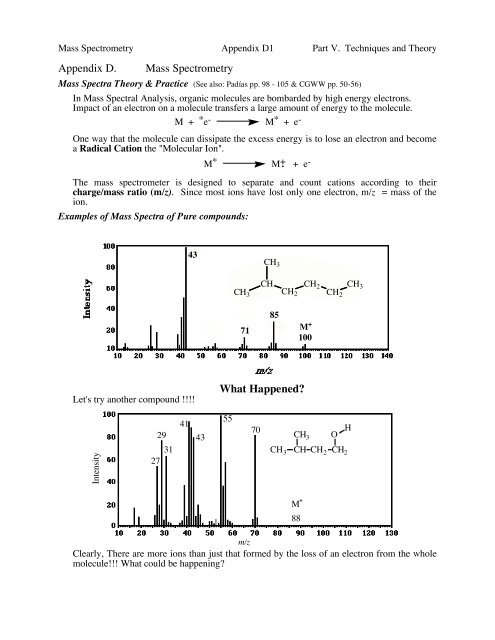
Examples of Mass Spectra of Pure compounds:<br />
43<br />
CH 3<br />
CH<br />
CH 3 CH 2<br />
CH 2<br />
CH2<br />
CH 3<br />
71<br />
85<br />
M +<br />
100<br />
Let's try another compound !!!!<br />
What Happened?<br />
Intensity<br />
27<br />
29<br />
31<br />
41<br />
43<br />
55<br />
70<br />
CH 3<br />
CH 3 O<br />
H<br />
CH CH 2 CH 2<br />
M +<br />
88<br />
m/z<br />
Clearly, There are more ions than just that formed by the loss of an electron from the whole<br />
molecule!!! What could be happening?