Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
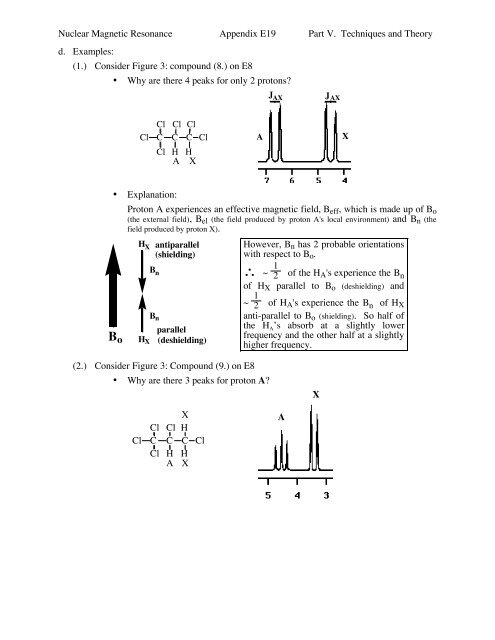
Nuclear Magnetic Resonance Appendix E19 Part V. Techniques and Theory<br />
d. Examples:<br />
(1.) Consider Figure 3: compound (8.) on E8<br />
• Why are there 4 peaks for only 2 protons?<br />
J AX<br />
J AX<br />
Cl Cl Cl<br />
Cl C C C Cl<br />
Cl H H<br />
A X<br />
A<br />
X<br />
• Explanation:<br />
B o<br />
Proton A experiences an effective magnetic field, B eff , which is made up of B o<br />
(the external field), B el (the field produced by proton A's local environment) and B n (the<br />
field produced by proton X).<br />
H X<br />
H X<br />
antiparallel<br />
(shielding)<br />
B n<br />
B n<br />
parallel<br />
(deshielding)<br />
However, B n has 2 probable orientations<br />
with respect to B o .<br />
∴ ~ 1<br />
2 of the H A's experience the B n<br />
of H X parallel to B o (deshielding) and<br />
~ 1<br />
2 of H A's experience the B n of H X<br />
anti-parallel to B o (shielding). So half of<br />
the H A ’s absorb at a slightly lower<br />
frequency and the other half at a slightly<br />
higher frequency.<br />
(2.) Consider Figure 3: Compound (9.) on E8<br />
• Why are there 3 peaks for proton A?<br />
X<br />
X<br />
Cl<br />
Cl<br />
C<br />
Cl<br />
C<br />
H<br />
C<br />
Cl H H<br />
A X<br />
Cl<br />
A