Anti-inflammatory Action of Erythromycin*
Anti-inflammatory Action of Erythromycin*
Anti-inflammatory Action of Erythromycin*
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
35<br />
C<br />
E<br />
U,<br />
0)<br />
U<br />
0<br />
0<br />
E<br />
C<br />
30<br />
25<br />
E<br />
0 I-<br />
C<br />
0<br />
U<br />
>.<br />
><br />
U,<br />
0)<br />
U,<br />
0<br />
x<br />
0<br />
I<br />
a-<br />
‘C<br />
z<br />
20<br />
15<br />
10<br />
Before<br />
After<br />
therapy<br />
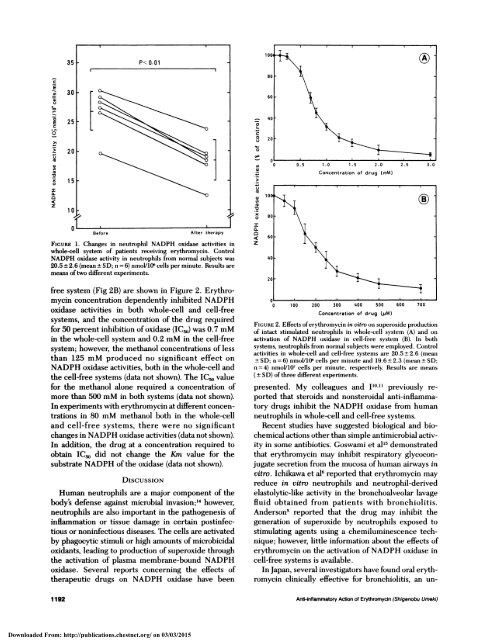
Ficuax 1. Changes in neutrophil NADPH oxidase activities in<br />
whole-cell system <strong>of</strong> patients receiving erythromycin. Control<br />
NADPH oxidase activity in neutrophils from normal subjects was<br />
20.5 ± 2.6 (mean ± SD; n = 6) nmol/10’ cells per minute. Results are<br />
means <strong>of</strong> two different experiments.<br />
1<br />
0<br />
0)0<br />
U,<br />
0)<br />
><br />
U<br />
U,<br />
5)<br />
U,<br />
U,<br />
#{149}0<br />
,; x<br />
0<br />
I<br />
a.<br />
0<br />
‘C<br />
z<br />
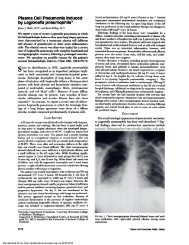
free system (Fig 2B) are shown in Figure 2. Erythromycin<br />
concentration dependently inhibited NADPH<br />
oxidase activities in both whole-cell and cell-free<br />
systems, and the concentration <strong>of</strong> the drug required<br />
for 50 percent inhibition <strong>of</strong> oxidase (IC30) was 0.7 mM<br />
in the whole-cell system and 0.2 mM in the cell-free<br />
system; however, the methanol concentrations <strong>of</strong> less<br />
than 125 mM produced no significant effect on<br />
NADPH oxidase activities, both in the whole-cell and<br />
the cell-free systems (data not shown). The IC30 value<br />
for the methanol alone required a concentration <strong>of</strong><br />
more than 500 mM in both systems (data not shown).<br />
In experiments with erythromycin at different concentrations<br />
in 80 mM methanol both in the whole-cell<br />
and cell-free systems, there were no significant<br />
changes in NADPH oxidase activities (data not shown).<br />
In addition, the drug at a concentration required to<br />
obtain IC30 did not change the Kin value for the<br />
substrate NADPH <strong>of</strong> the oxidase (data not shown).<br />
DISCUSSION<br />
Human neutrophils are a major component <strong>of</strong> the<br />
body’s defense against microbial invasion;’4 however,<br />
neutrophils are also important in the pathogenesis <strong>of</strong><br />
inflammation or tissue damage in certain postinfectious<br />
or noninfectious diseases. The cells are activated<br />
by phagocytic stimuli or high amounts <strong>of</strong> microbicidal<br />
oxidants, leading to production <strong>of</strong> superoxide through<br />
the activation <strong>of</strong> plasma membrane-bound NADPH<br />
oxidase. Several reports concerning the effects <strong>of</strong><br />
therapeutic drugs on NADPH oxidase have been<br />
0 100 200 300 ‘400 500 600<br />
Concentration <strong>of</strong> drug (hiM)<br />
FIGURE 2. Effects <strong>of</strong>erythromycin in vitro on superoxide production<br />
<strong>of</strong> intact stimulated neutrophils in whole-cell system (A) and on<br />
activation <strong>of</strong> NADPH oxidase in cell-free system (B). In both<br />
systems, neutrophils from normal subjects were employed. Control<br />
activities in whole-cell and cell-free systems are 20.5 ± 2.6 (mean<br />
± SD; n = 6) nmol/10’ cells per minute and 19.6 ± 2.3 (mean ± SD;<br />
n = 4) nmol/10 cells per minute, respectively. Results are means<br />
(± SD) <strong>of</strong>three different experiments.<br />
presented. My colleagues and U0,” previously reported<br />
that steroids and nonsteroidal anti-<strong>inflammatory</strong><br />
drugs inhibit the NADPH oxidase from human<br />
neutrophils in whole-cell and cell-free systems.<br />
Recent studies have suggested biological and biochemical<br />
actions other than simple antimicrobial activity<br />
in some antibiotics. Goswami et al’5 demonstrated<br />
that erythromycin may inhibit respiratory glycoconjugate<br />
secretion from the mucosa <strong>of</strong> human airways in<br />
vitro. Ichikawa et al6 reported that erythromycin may<br />
reduce in vitro neutrophils and neutrophil-derived<br />
elastolytic-like activity in the bronchoalveolar lavage<br />
fluid obtained from patients with bronchiolitis.<br />
Anderson5 reported that the drug may inhibit the<br />
generation <strong>of</strong> superoxide by neutrophils exposed to<br />
stimulating agents using a chemiluminescence technique;<br />
however, little information about the effects <strong>of</strong><br />
erythromycin on the activation <strong>of</strong> NADPH oxidase in<br />
cell-free systems is available.<br />
In Japan, several investigators have found oral erythromycin<br />
clinically effective for bronchiolitis, an Un-<br />
700<br />
1192 <strong>Anti</strong>-<strong>inflammatory</strong> <strong>Action</strong> <strong>of</strong> Erythromycin (Shigenobu Umeki)<br />
Downloaded From: http://publications.chestnet.org/ on 03/03/2015