Daniil Itkis Lomonosov Moscow State University - elch.chem.msu.ru
Daniil Itkis Lomonosov Moscow State University - elch.chem.msu.ru
Daniil Itkis Lomonosov Moscow State University - elch.chem.msu.ru
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Model<br />
D = 2RT Development<br />
efft + − 1 f<br />
1 +<br />
F ln c Li 8<br />
Basic equations.— The mathematical Model description Development of Li-air bateries<br />
should include accurate models for the lithium-ion and oxygen<br />
iffusion absolute inside the temperature. cell, theInelectron our simulations, conductivity f / of ln cthe Li iscarbon<br />
approximated<br />
Li 2 to O 2 zero. formation and deposition at the cathode, the po-<br />
athode, the<br />
osity change inside the cathode, and rate equations for Reaction 1<br />
nd the Li-ion formation Li → Li + +e − . In this work, we used the<br />
heory<br />
ude.<br />
of concentrated solutions 10 to model the Li + and oxygen difusion<br />
and drift in the anode protective layer APL, separator, and<br />
Model Development<br />
athode electrolyte. 3.0 We also assumed a binary monovalent electroyte<br />
and no convection. The electrostatic potential of Li ions Li is<br />
2.8<br />
ssumed to satisfy the following drift-diffusion equation<br />
O2 c O2<br />
ref<br />
=2k<br />
r¯pc O2<br />
e 1−F/RT c − e −F/RT c if LC x<br />
where<br />
Basic<br />
R =<br />
equations.—<br />
8.314 J/mol KThe is themathematical universal gas constant<br />
description<br />
and T is theof Li-air bat-<br />
First theoretical models<br />
0 otherwise<br />
clude.<br />
C<br />
iffusion and reaction rate at the cathode. The formation of Li 2 O 2 at<br />
e cathode is modeled similarly to the one developed by Sandhu et<br />
. 6 In the second section, we present the numerical algorithm and<br />
mple simulation results. Then, we discuss about possible aproaches<br />
to improve the specific capacity SC of the cathode electeries<br />
should include accurate models for the lithium-ion and oxygen<br />
diffusion inside the cell, the electron conductivity of the carbon<br />
where = 0.5, k is a reaction rate constant, c<br />
ode and the energy density of Li-air batteries, after which we con-<br />
O2<br />
cathode, the Li 2 O 2 formation and deposition at the cathode, normalization the porosity<br />
change inside the cathode, Journal of and The Electro<strong>chem</strong>ical rate equations Society, for 157 12 Reaction IfA1287-A1295 we take1into 2010 consideration the electrical A1291 resistivity<br />
parameter, and c is the overpotential a<br />
which is assumed to deposit uniformly on the inner<br />
and the Li-ion formation Li → Li + +e − . In this work, we<br />
0.8 pores,<br />
usedthe theoverpotential can be written as<br />
theory of concentrated solutions 10 to model the Li<br />
Basic equations.— The mathematical description of Li-air + and oxygen diffusion<br />
and drift in the anode protective layer APL, separator, and<br />
2<br />
batries<br />
should include accurate models for the lithium-ion and oxygen<br />
c = Li − − U c − V Li2 O<br />
cathode electrolyte. <br />
0.6<br />
iffusion inside the 2.6 We also assumed a binary monovalent electro-<br />
· cell, the electron conductivity of the carbon = Li − − U c − R C eff Li + D ln c Li − R C =0 2<br />
Li2 O 2<br />
r¯p,0<br />
0<br />
ln<br />
I=0.1mA/cm 2<br />
lyte and no convection. The electrostatic potential of Li ions <br />
thode, the Li 2 O 2 formation and deposition at the cathode, the posity<br />
change inside the cathode, and rate equations for Reaction 1<br />
Li is<br />
here eff is the effective electric conductivity of the electrolyte, D<br />
lectrolyte d the Li-ion which formation is equal toLi the→concentration Li + +e − . of InLi this + , and work, R C iswe theused the<br />
eory xygenofconversion concentrated rate, solutions which is 10 equal to model to zerothe in Li the + APL and oxygen and difsion<br />
and<br />
eparator and<br />
drift<br />
is positive<br />
in the anode<br />
in the cathode.<br />
protective<br />
The<br />
layer<br />
concentration<br />
APL, separator,<br />
of Li +<br />
and<br />
atisfies<br />
thode 10<br />
electrolyte. We also assumed a binary monovalent electrote<br />
and no convection. The electrostatic potential of Li ions Li is<br />
sumed to satisfy the following drift-diffusion equation<br />
Voltage (V)<br />
2.4<br />
assumed to satisfy the following drift-diffusion (e) equation where V Li2 O 2<br />
is the voltage drop across Li 2 O 2 , Li2<br />
0.4<br />
2.2<br />
(a)(b)<br />
(c) (d)<br />
trical resistivity 0capacity of Li 2 O 2 , and U c is the equilibrium<br />
2.9<br />
· 0.05 mA/cm 2<br />
Reaction 1 at the cathode.<br />
2.8 eff Li + <br />
(e) D ln c Li − R C =0 2 1/4 capacity<br />
2.0<br />
2.7<br />
(d) 0.1 mA/cm 2<br />
Equations 2-4, 2/4 capacity 9, 10, and 12 represent a system of<br />
(c)<br />
where eff is the effective 2.6 electric conductivity 0.2 mA/cm 2 of the electrolyte, 0.2 ential equations <br />
(b)<br />
D 3/4that capacity should be subject to boundary a<br />
2.5<br />
1.8<br />
(a)<br />
is the diffusional conductivity, c 0.5 mA/cm 2<br />
ditions and should 4/4 capacity be solved self-consistently to<br />
2.4<br />
0.0 0.1 0.2 0.3 0.4 0.5 Li is the concentration of the lithium<br />
lithium-ion and oxygen concentrations, the electrosta<br />
electrolyte<br />
Specific capacity (mAh/g C<br />
)<br />
1mA/cm<br />
c which is equal to the concentration 2<br />
of Li + Li <br />
, and<br />
1.6<br />
0.0 and<br />
R C the<br />
is the<br />
porosity at each location inside the electroche<br />
oxygen= conversion · D0 Li,eff 200 rate, c Li 400 which − 1 − 600 t+<br />
800 1000 1200<br />
0.0 function of 0.2 time. The 0.4 initial 0.6 conditions0.8<br />
t<br />
F<br />
is R C equal − I Li · t +<br />
to zero3<br />
F<br />
in the APL and<br />
as well as<br />
Distance (mm)<br />
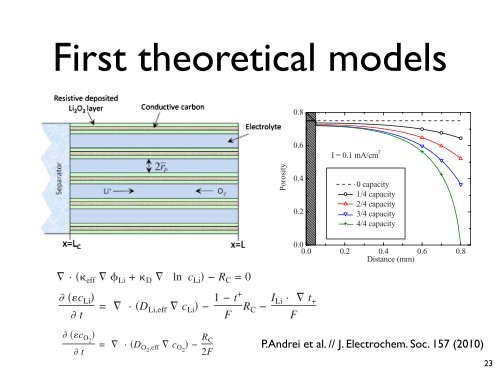
separator Figure 2. Color and is online positive Modeling Specific inof capacity the oxygen cathode. (mAh/g diffusion C<br />
) The and concentration Li 2 O 2 formation<br />
in the porous carbon cathode.<br />
presented in the next two<br />
conditions of Li + for the one-dimensional cell simulated in<br />
subsections.<br />
s the diffusional conductivity, c Li is the concentration of the lithium<br />
Voltage (V)<br />
here is the porosity, D Li,eff is the effective electrolyte diffusion<br />
oefficient, t + is the transference number, F = 96,487 C/mol is<br />
satisfies 10 · eff Li + D ln c Li − R C =0 2<br />
Figure 4. Color online Cell voltage as a function of the SC for different<br />
discharge currents.<br />
here araday’s eff constant, is the c effective and Li I Li electric = − eff conductivity Li − D of the electrolyte, D<br />
the diffusional conductivity, = · D c Li Li,eff is the c Li − 1 ln − c t+ Li<br />
of lithium<br />
t<br />
F R is the<br />
C − I Li · t +<br />
lectrolyte density current. The oxygen concentration satisfies the 3<br />
ollowing<br />
ectrolytediffusion which<br />
equation<br />
is equal to the concentration of Li + F<br />
, and R C is the<br />
xygenwhere conversion is therate, porosity, whichDis Li,eff equal is the to effective zero in the electrolyte APL andiffusion<br />
parator coefficient, and is positive t + in the cathode. The concentration of Li +<br />
tisfies Faraday’s 10 constant, and I Li = − eff Li − D ln c Li is the<br />
maximum limit given by Eq. 27 because, for narrow cathodes, the one should either increase the diffusion coefficient of O 2 to make it<br />
oxygen can diffuse completely throughout the whole cathode volume.<br />
The02 cell Feb voltage 2011 decreases to 195.208.208.29. with decreasing Redistribution thickness of the subject side of to the ECS cathode license near the or copyright; separator to efficiently see http://www.ecsdl.org/t<br />
fill in the pores<br />
comparable to that of Li ions or increase the reaction rate at the left<br />
Downloaded c<br />
cathode O2 <br />
electrode. This is because in batteries with smaller cathode of the cathode with Li 2 O 2 .<br />
thickness, there<br />
= is is the less· surface<br />
Dtransference O2 ,eff areaavailable c O2 −and number, R C<br />
the overpotential F = atP.Andrei 4 96,487 The dependence et C/mol al. // of J. is the Electro<strong>chem</strong>. reaction rate as a function Soc. of 157 the position (2010)<br />
t<br />
2F<br />
the cathode as expressed by Eq. 14 becomes higher. The SC in Fig. inside the cell is represented in Fig. 7 for different states of discharge.<br />
While the battery discharges, the reaction rate decreases 23<br />
4 and 5 as well as in other figures in this section is expressed in<br />
mAh/g , where the mass includes only the mass of the carbon in the deep inside the cathode and increases at the surface of the cathode.<br />
here D O ,eff is the effective diffusion constant of the oxygen.<br />
Porosity<br />
Figure 6. Color online Local dependence of the porosity when the battery<br />
is fully charged 0 capacity, partly charged 1/4, 2/4, and 3/4 capacity, and<br />
nearly completely discharged 4/4 capacity.<br />
ref =