Thesis for degree: Licentiate of Engineering
Thesis for degree: Licentiate of Engineering
Thesis for degree: Licentiate of Engineering
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
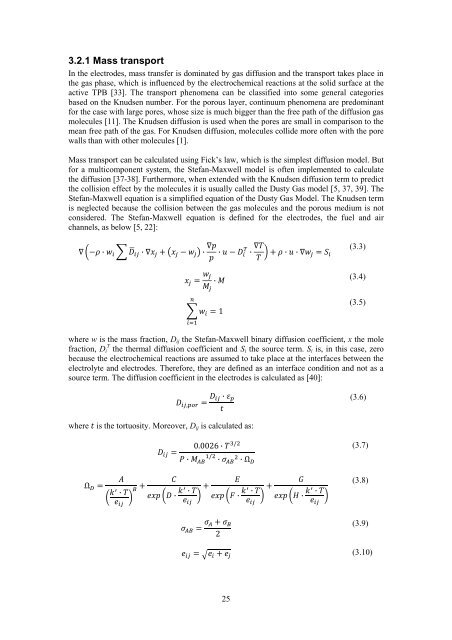
3.2.1 Mass transport<br />
In the electrodes, mass transfer is dominated by gas diffusion and the transport takes place in<br />
the gas phase, which is influenced by the electrochemical reactions at the solid surface at the<br />
active TPB [33]. The transport phenomena can be classified into some general categories<br />
based on the Knudsen number. For the porous layer, continuum phenomena are predominant<br />
<strong>for</strong> the case with large pores, whose size is much bigger than the free path <strong>of</strong> the diffusion gas<br />
molecules [11]. The Knudsen diffusion is used when the pores are small in comparison to the<br />
mean free path <strong>of</strong> the gas. For Knudsen diffusion, molecules collide more <strong>of</strong>ten with the pore<br />
walls than with other molecules [1].<br />
Mass transport can be calculated using Fick’s law, which is the simplest diffusion model. But<br />
<strong>for</strong> a multicomponent system, the Stefan-Maxwell model is <strong>of</strong>ten implemented to calculate<br />
the diffusion [37-38]. Furthermore, when extended with the Knudsen diffusion term to predict<br />
the collision effect by the molecules it is usually called the Dusty Gas model [5, 37, 39]. The<br />
Stefan-Maxwell equation is a simplified equation <strong>of</strong> the Dusty Gas Model. The Knudsen term<br />
is neglected because the collision between the gas molecules and the porous medium is not<br />
considered. The Stefan-Maxwell equation is defined <strong>for</strong> the electrodes, the fuel and air<br />
channels, as below [5, 22]:<br />
(3.3)<br />
(3.4)<br />
(3.5)<br />
where w is the mass fraction, D ij the Stefan-Maxwell binary diffusion coefficient, x the mole<br />
fraction, D i T the thermal diffusion coefficient and S i the source term. S i is, in this case, zero<br />
because the electrochemical reactions are assumed to take place at the interfaces between the<br />
electrolyte and electrodes. There<strong>for</strong>e, they are defined as an interface condition and not as a<br />
source term. The diffusion coefficient in the electrodes is calculated as [40]:<br />
(3.6)<br />
where is the tortuosity. Moreover, D ij is calculated as:<br />
(3.7)<br />
(3.8)<br />
(3.9)<br />
(3.10)<br />
25