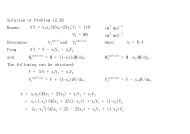K = K0 K1 K2 K0 K1 K2
K = K0 K1 K2 K0 K1 K2
K = K0 K1 K2 K0 K1 K2
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Solution to Problem 13.12<br />
Known: For reaction N 2 (g) + C 2 H 2 (g) → 2HCN(g)<br />
Initial 1 mol 1 mol 0 mol 0 mol<br />
Ideal gas t = 650 ℃ T = 923.15 K 1 bar<br />
Determine the composition of the system at equilibrium<br />
From<br />
K = K 0 K 1 K 2<br />
K 0 = exp(-ΔG o 0 /RT 0 )<br />
K 1 = exp((ΔH o 0 /RT 0 )(1-T 0 /T))<br />
K 2 = exp((-1/T)∫(ΔC o p /R)dT + ∫(ΔC o p /R)(dT/T))<br />
= exp{ΔA[lnτ-(τ-1)/τ] + (1/2)ΔBT 0 (τ-1) 2 /τ + (1/6)ΔCT 2 0 (τ-1) 2 (τ+2)/τ + (1/2)ΔD(τ-1) 2 /(T 2 0 τ 2 )}<br />
ΔA = 2*4.736 - 3.280 - 6.132 = 0.060000<br />
ΔB = (2*1.359 - 0.593 - 1.952)*0.001 = 0.000173 1.730E-04<br />
ΔC = 0<br />
ΔD = (-2*0.725 - 0.040 + 1.299) * 100000 = -19100 -1.910E+04
ΔH o 298 = 2*135100 - 227480 = 42720.00 J mol -1<br />
ΔG o 298 = 2*124700 - 209970 = 39430.00 J mol -1<br />
ΔG o /RT =<br />
K 0 = exp(-ΔG o 0 /RT 0 )<br />
= exp(-39430/(8.314*298.15))<br />
= 1.2353E-07<br />
K 1 = exp((ΔH o 0 /RT 0 )(1-T 0 /T))<br />
= exp((42720/(8.314*298.15))*(1-298.15/923.15))<br />
= 1.1677E+05<br />
τ = 923.15/298.15 = 3.0963<br />
K 2 = exp{ΔA[lnτ-(τ-1)/τ] + (1/2)ΔBT 0 (τ-1) 2 /τ + (1/6)ΔCT 2 0 (τ-1) 2 (τ+2)/τ + (1/2)ΔD(τ-1) 2 /(T 2 0 τ 2 )}<br />
= exp(0.06*(ln(3.0963)-(3.0963-1)/3.0963)+(1/2)*0.000173*298.15*(3.0963-1)*(3.0963-1)/3.0963<br />
+(1/2)*(-19100)*(3.0963-1)*(3.0963-1)/(298.15*298.15*3.0963*3.0963))<br />
= 1.0147<br />
K = K 0 K 1 K 2<br />
= 1.2353*1.1677*1.0147E-2<br />
= 1.4637E-02
Set ε as the reaction coordinate of the system at equilibrium<br />
K = 4ε 2 /[(1-ε)(1-ε)]<br />
or 4ε 2 /[(1-ε)(1-ε)] - K = 0<br />
By trial and error<br />
From<br />
K = 4ε 2 /[(1-ε)(1-ε)]<br />
and<br />
K = 0.014637<br />
We can get<br />
ε<br />
4ε 2 /[(1-ε)(1-ε)] - K<br />
0.2 0.23536300<br />
0.1 0.03474572<br />
0.05 -0.00355667<br />
0.06 0.00165997<br />
0.055 -0.00108755<br />
0.057 -0.00002242<br />
0.0575 0.00025088<br />
0.0572 0.00008656
0.0571 0.00003201<br />
0.05705 0.00000478 0.0571<br />
N 2 (g) + C 2 H 2 (g) → 2HCN(g)<br />
So N 2 (g) = 1-ε 0.9429 mol<br />
C 2 H 2 (g) = 1-ε 0.9429 mol<br />
2HCN(g) = 2ε 0.1142 mol<br />
2 2<br />
y N2 = (1-0.0571)/2 = 0.4715<br />
y C2H2 = (1-0.0571)/2 = 0.4715<br />
y HCN = 2*0.0571/2 = 0.0571<br />
1.0000