The Heck Reaction: Mechanistic Insight into a ... - The Stoltz Group
The Heck Reaction: Mechanistic Insight into a ... - The Stoltz Group
The Heck Reaction: Mechanistic Insight into a ... - The Stoltz Group
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>The</strong> <strong>Heck</strong> <strong>Reaction</strong>: <strong>Mechanistic</strong> <strong>Insight</strong> <strong>into</strong><br />
a Synthetically Useful <strong>Reaction</strong><br />
Uttam K. Tambar<br />
<strong>Stoltz</strong> <strong>Group</strong> Literature Series<br />
Thursday, April 17, 2003 (right after group meeting)<br />
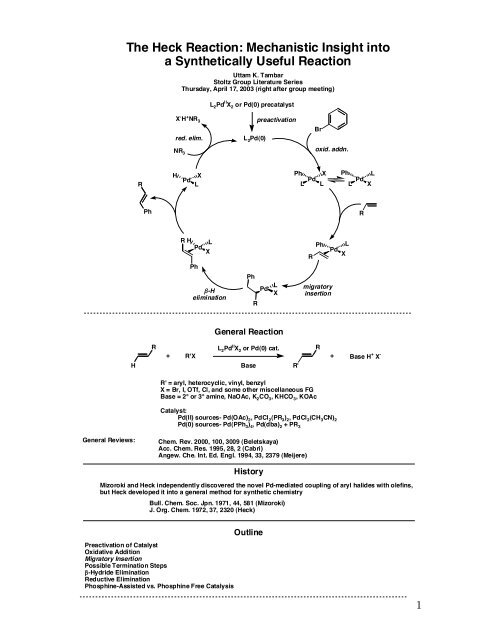
L 2 Pd II X 2 or Pd(0) precatalyst<br />
X - H + NR 3<br />
red. elim.<br />
NR 3<br />
L 2 Pd(0)<br />
preactivation<br />
Br<br />
oxid. addn.<br />
R<br />
H<br />
Pd<br />
X<br />
L<br />
Ph<br />
L<br />
Pd<br />
L<br />
X<br />
Ph<br />
L<br />
Pd<br />
X<br />
L<br />
Ph<br />
R<br />
R H<br />
Pd<br />
X<br />
L<br />
R<br />
Ph<br />
Pd<br />
X<br />
L<br />
Ph<br />
Ph<br />
!-H<br />
elimination<br />
R<br />
Pd<br />
L X<br />
migratory<br />
insertion<br />
General <strong>Reaction</strong><br />
R<br />
+ R'X<br />
L 2 Pd II X 2 or Pd(0) cat.<br />
R<br />
+ Base H + X -<br />
H<br />
Base<br />
R'<br />
R' = aryl, heterocyclic, vinyl, benzyl<br />
X = Br, I, OTf, Cl, and some other miscellaneous FG<br />
Base = 2° or 3° amine, NaOAc, K 2 CO 3 , KHCO 3 , KOAc<br />
Catalyst:<br />
Pd(II) sources- Pd(OAc) 2 , PdCl 2 (PR 3 ) 2 , PdCl 2 (CH 3 CN) 2<br />
Pd(0) sources- Pd(PPh 3 ) 4 , Pd(dba) 2 + PR 3<br />
General Reviews:<br />
Chem. Rev. 2000, 100, 3009 (Beletskaya)<br />
Acc. Chem. Res. 1995, 28, 2 (Cabri)<br />
Angew. Che. Int. Ed. Engl. 1994, 33, 2379 (Meijere)<br />
History<br />
Mizoroki and <strong>Heck</strong> independently discovered the novel Pd-mediated coupling of aryl halides with olefins,<br />
but <strong>Heck</strong> developed it <strong>into</strong> a general method for synthetic chemistry<br />
Bull. Chem. Soc. Jpn. 1971, 44, 581 (Mizoroki)<br />
J. Org. Chem. 1972, 37, 2320 (<strong>Heck</strong>)<br />
Preactivation of Catalyst<br />
Oxidative Addition<br />
Migratory Insertion<br />
Possible Termination Steps<br />
!-Hydride Elimination<br />
Reductive Elimination<br />
Phosphine-Assisted vs. Phosphine Free Catalysis<br />
Outline<br />
1
Preactivation of Catalyst<br />
• if a Pd(II) source is used in a <strong>Heck</strong> reaction, it must be reduced to Pd(0) before entering the catalytic cycle<br />
Reduction by Phosphines<br />
outer shell mechanism<br />
nucleophillic<br />
attack<br />
H 2 O<br />
L n Pd II PR 3<br />
L n Pd(O) + NuPR 3<br />
Nu<br />
H<br />
+<br />
O PR 3<br />
Nu<br />
phosphonium<br />
inner shell mechanism<br />
Nu<br />
reductive<br />
elimination<br />
H 2 O<br />
L n Pd II PR 3<br />
L n Pd(O) + NuPR 3<br />
Nu<br />
H<br />
+<br />
O PR 3<br />
phosphonium<br />
• electron-withdrawing group on phosphine increases rate of Pd(II) to Pd(0) reduction,<br />
because phosphine is more susceptible to attack by nucleophile<br />
• hard nucleophiles assist Pd(II) to Pd(0) reduction (eg. - OH, - OR, H 2 O, AcO - /H 2 O, F - /H 2 O)<br />
Preactivation of Catalyst - continued<br />
• in the absence of phosphines, Pd(II) can be reduced to Pd(0) by other means<br />
Reduction by Tertiary Aliphatic Amines<br />
L n Pd II<br />
X<br />
X<br />
coordination<br />
R 2 N<br />
R<br />
L<br />
X<br />
R 2<br />
N<br />
Pd<br />
H<br />
R<br />
!-hydride<br />
elimination<br />
R 2 N<br />
R<br />
L n Pd II<br />
X<br />
H<br />
reductive<br />
elimination<br />
HX<br />
L n Pd(0)<br />
Reduction by Olefins (Wacker-Type Process)<br />
L n Pd II<br />
X<br />
X<br />
coordination<br />
R<br />
L n Pd II<br />
R<br />
X<br />
Wacker-type<br />
attack<br />
Nu<br />
H<br />
L n Pd II<br />
X<br />
Nu<br />
R<br />
!-hydride<br />
elimination<br />
R<br />
L n Pd II<br />
X<br />
H<br />
reductive<br />
elimination<br />
HX<br />
L n Pd(0)<br />
Nu<br />
• when a high catalyst loading is used, the yield may be diminished if the substrate olefin<br />
undergoes this Wacker-type reaction<br />
Reduction by Other Functional <strong>Group</strong>s<br />
• phosphonium and 4° ammonium salts can also reduce Pd(II) to Pd(0) (via oxid. addn. to C-N or C-P)<br />
2
Preactivation of Catalyst - continued<br />
• to enter the catalytic cycle, Pd(0) must have a proper coordination shell (eg. L 2 Pd(0) )<br />
there can be no more than 2 strongly bound ligands on palladium<br />
there is a a restriction on the choice of ligands and their concentration in solution<br />
<strong>The</strong> Proper Coordination Shell for Pd(0) in Monodentate Phosphine Assited Catalysis<br />
• too much phosphine inhibits catalysis by generating a coordinatively saturated complex:<br />
PR 3<br />
R 3 P Pd 0 PR 3<br />
PR 3<br />
R 3 P Pd 0 PR 3<br />
Ar Br<br />
PR 3 ligand<br />
ox. addn.<br />
assoc.<br />
Ar<br />
R 3 P Pd II Br<br />
PR 3<br />
<strong>Heck</strong><br />
• but with too little phosphine, the active dicoordinated Pd 0 (PR 3 ) 2 disproportionates<br />
to a stable tricoordinate Pd 0 (PR 3 ) 3 and unstable low-ligated complexes, which leads<br />
to Pd aggregation and cluster formation:<br />
2Pd(PR 3 ) 2 Pd(PR 3 ) 3 + Pd(PR 3 ) Pd n L m Pd-black<br />
(stable)<br />
Preactivation of Catalyst - continued<br />
<strong>The</strong> Proper Coordination Shell for Pd(0) Complexes Generated from Pd(dba) 2<br />
• once again, with too little phosphine (or other L-type ligand) the active dicoordinated Pd 0 L 2<br />
disproportionates to a stable tricoordinate Pd 0 L 3 and unstable low-ligated complexes, which<br />
leads to Pd aggregation and cluster formation:<br />
Pd(dba) 2<br />
+ 2L 2dba + PdL 2 PdL 3 + PdL Pd n L m Pd-black<br />
• with too little phosphine there may also be incomplete deligation of dba,<br />
which requires at least 4 equivalents of phosphine<br />
• Pd(dba) 2 + excess PR 3 may lead to a coordinatively saturated complex,<br />
which may not react with less reactive substrates<br />
preformed Pd(PPR 3 ) 4 may be more reactive thanPd(dba) 2 + excess PR 3<br />
Electronic Influences on the Proper Coordination Shell for Pd(0)-Triaryl Phosphine Complexes<br />
• there's a fine balance in choosing a triaryl phosphine ligand with the proper electronices<br />
electron withdrawing<br />
group on arylphosphine<br />
higher concentration of reactive Pd 0 (PAr 3 ) 2 via deligation of PAr 3<br />
electron donating<br />
group on arylphospine<br />
higher rate of oxidative addition<br />
3
Oxidative Addition<br />
L 2 Pd(0)<br />
oxid. addn.<br />
Ph<br />
L<br />
Pd<br />
L<br />
X<br />
Ph<br />
L<br />
Pd<br />
X<br />
L<br />
Br<br />
cis<br />
trans<br />
• oxidative addition is a relatively concerted process (C-X breaks synchronously with the formation<br />
of M-C and M-X)<br />
• unlike the stepwise addition-elimination mechanism of nucleophillic aromatic substitution (in which<br />
charge builds up), the concerted mechanism of oxidative addition is less sensitive to the electronics<br />
of the unsaturated R'-X substrate and is more sensitive to the nature of X and the relative bond<br />
strengths of C-X and M-X<br />
order of reactivity in oxidative addition (I >> OTf > Br >> Cl) is roughly<br />
opposite for nucleophillic aromatic substitution<br />
• in most monodentate phosphine examples, the isolable product of oxidative addition is the trans isomer,<br />
but the complex that continues in the catalytic cycle is the cis isomer<br />
<strong>Heck</strong> Ph X Ph L<br />
Pd<br />
Pd<br />
isolable<br />
L L L X<br />
cis<br />
trans<br />
R X<br />
L<br />
Pd<br />
L<br />
Migratory Insertion<br />
migratory<br />
insertion<br />
R'<br />
R<br />
R'<br />
L<br />
Pd<br />
X<br />
L<br />
• this step is usually responsible for the regioselectivity, stereoselectivity and substrate selectivity<br />
or a <strong>Heck</strong> reaction<br />
3 Possible Mechanisms<br />
(1) RPdX behaves as a carbanion (like other non-transition or early-transition metals)<br />
R<br />
L<br />
Pd<br />
L<br />
X<br />
Pd<br />
L<br />
X<br />
L<br />
R<br />
O<br />
OMe<br />
migratory<br />
insertion<br />
R<br />
Pd<br />
L<br />
O<br />
X<br />
L<br />
OMe<br />
most data refutes this mechanism<br />
(2) RPdX or RPd + are metal-centered electrophiles being attacked by an olefin<br />
(electrophillic addition mechanism)<br />
X<br />
L<br />
R<br />
Pd<br />
X<br />
L<br />
Pd<br />
R<br />
X<br />
L<br />
Pd<br />
R<br />
there is some data that supports this mechanism, and<br />
sometimes it is the most relevant mechanism<br />
4
Migratory Insertion - continued<br />
(3) RPdX or RPd + add to the olefin in a concerted process<br />
X<br />
L<br />
Pd<br />
R<br />
X<br />
L<br />
Pd<br />
R<br />
X<br />
L<br />
Pd<br />
R<br />
this mechanism is the most realistic (compromise between the 2<br />
other possible mechisms)<br />
the variable TS is adaptable/flexible to electronic demands, so<br />
steric factors are often the main source of selectivitiy<br />
eg. substitution on olefin reduces rate of reaction<br />
> ><br />
Migratory Insertion - continued<br />
Mechanism of Olefin Ligation to Pd(II)<br />
• in order for the olefin to ligate to Pd(II), another ligand must deligate first to open a coordination site<br />
if a neutral L ligand deligates (eg. PR 3 )<br />
non-polar / neutral mechanism<br />
if an anionic X ligand deligates (eg. halide)<br />
polar / cationic mechanism<br />
• neutral mechanism:<br />
L 2 Pd(0)<br />
X<br />
Ar<br />
Ar<br />
L<br />
Pd<br />
L<br />
X<br />
R<br />
R<br />
Ar<br />
Pd<br />
L<br />
X<br />
L<br />
this mechanism is thought to operate when X is a strong !-donor (ie. Cl, Br, I)<br />
• cationic mechanism:<br />
+<br />
X Ar<br />
Ar L<br />
L<br />
Ar L<br />
2 Pd(0)<br />
R<br />
Pd<br />
Pd<br />
X L<br />
R<br />
L<br />
Cl -<br />
X -<br />
this mechanism is thought to operate when X is a weakly associated anionic ligand<br />
(eg. OTf, OAc), or when Ag or Tl salts (eg. AgY or TlY, Y =CO 3 , OTf, OAc)<br />
are used to abstract the halide<br />
5
Migratory Insertion - continued<br />
• for monodentate phosphine ligands, both the neutral and cationic mechanisms are accessible:<br />
neutral<br />
Ar<br />
L Pd L<br />
X<br />
cationic<br />
-L<br />
L<br />
Ar<br />
Pd S L<br />
Ar<br />
Pd<br />
X<br />
X<br />
L<br />
Ar<br />
Pd X<br />
-X - L<br />
Ar<br />
Pd S<br />
L<br />
Ar<br />
Pd<br />
L<br />
L<br />
L<br />
• for bidentate phosphine ligands, the neutral mechanism a lot less likely<br />
when aryl or vinyl halides are used, bidentate ligands often lead to a complete<br />
or partial suppression of the reaction<br />
the neutral mechanism is still possible in special cases<br />
eg. large bite-angle diphosphine in which the spacer between phosphines<br />
is large/flexible and the P-Pd-P angle is greater than 90 °<br />
neutral (less likely)<br />
L<br />
Ar<br />
Pd X<br />
L<br />
cationic<br />
-L<br />
S<br />
Ar<br />
Pd X<br />
L L<br />
Ar<br />
-X - L Pd S<br />
L<br />
L<br />
L<br />
Ar<br />
Pd<br />
L<br />
Ar<br />
Pd<br />
L<br />
X<br />
Migratory Insertion - continued<br />
• for phosphine-free systems, the neutral mechanism is bascially inconceivable<br />
most phosphine-free <strong>Heck</strong> reactions are performed in polar solvents or with<br />
additives which assist in the exchange of anionic ligands<br />
• the electrophillicity of the arylpalladium complex has little to do with the positive charge of Pd<br />
ArPdX / ArPd + is electrophillic with electron rich olefins<br />
ArPdX / ArPd + is nucleophillic with electron poor olefins<br />
ArPd + is not more electrophillic than ArPdX<br />
• there are several degrees of freedom in the TS<br />
the !-complexed alkene rotates to an in-plane position and therefore adapts<br />
itself to 2 possible TS (representing the 2 possible regioisomeric products of<br />
olefin insertion)<br />
the TS which represents the lower energy barrier is followed<br />
6
Regioselectivity in Migratory Insertion<br />
• "the temptation to find simple rules of regioselectivity should be discarded"<br />
Intermolecular <strong>Heck</strong> <strong>Reaction</strong><br />
• regioselectivity of migratory insertion with neutral Pd complexes<br />
20%<br />
80%<br />
CO 2 Me CN Ph C 4 H 9<br />
1%<br />
40%<br />
99%<br />
60%<br />
CO 2 Me Ph N<br />
O<br />
OMe<br />
neutral Pd complex + electron poor alkene<br />
neutral Pd complex + electron rich alkene<br />
steric control<br />
electronic control<br />
Regioselectivity in Migratory Insertion - continued<br />
• regioselectivity of migratory insertion with cationic Pd complexes<br />
40%<br />
80%<br />
60%<br />
20%<br />
CO 2 Me CN Ph C 4 H 9<br />
95%<br />
O<br />
N<br />
OH<br />
5%<br />
OAc<br />
OAc<br />
cationic Pd complex + electron poor alkene<br />
cationic Pd complex + electron rich alkene<br />
electronic control<br />
electronic control<br />
coordination of the olefin to<br />
a cationic Pd complex results<br />
in a polarization of the C=C bond<br />
7
Regioselectivity in Migratory Insertion<br />
Intramolecular <strong>Heck</strong> <strong>Reaction</strong><br />
• regioselectivity of intramolecular <strong>Heck</strong> reactions is almost always determined by sterics<br />
exo-trig<br />
endo-trig<br />
Pd<br />
L X<br />
PdL n X<br />
Pd<br />
L X PdL n X<br />
for small rings (5, 6, or 7 carbons)<br />
exo-trig dominates<br />
for large rings / macrocycles (more than 9 carbons)<br />
endo-trig is generally preferred<br />
• disfavored regioselectivity in intermolecular <strong>Heck</strong> reactions can be<br />
realized by using a cleavable tether to furnish a desirable exo-trig product (TL 1999, 40, 4901)<br />
X<br />
I<br />
+<br />
OMe<br />
X<br />
I<br />
OMe<br />
X<br />
MeO<br />
X<br />
MeO<br />
Y<br />
OBn<br />
RO 2 C<br />
Y<br />
O<br />
O<br />
Y<br />
<strong>Heck</strong><br />
O<br />
O<br />
Y<br />
OBn<br />
CO 2 R<br />
Termination Step<br />
• after migratory insertion and before reductive elimination there are several options for transforming the<br />
organopalladium complex<br />
(1) Pd-H elimination / !-hydride elimination (this is the most common)<br />
(2) Pd-M elimination (eg. Pd-SiX 3 )<br />
(3) palladotropic shift: Ar Pd X<br />
O<br />
OMe<br />
XPd<br />
O<br />
OMe<br />
XPd<br />
O<br />
OMe<br />
Ar<br />
if Pd-H elimination is not possible because of stereochemical reasons or is very slow,<br />
Ar<br />
(4) nucleophillic attack at Pd (nucleophillic substitution or reductive elimination),<br />
which results in a net syn Ar-Nu addition across an olefin<br />
(5) cascade reactions: allylic substitution<br />
cross coupling<br />
carbonylation<br />
another <strong>Heck</strong><br />
8
!-Hydride Elimination<br />
Ph<br />
Pd<br />
L X<br />
R H<br />
Pd<br />
L<br />
X<br />
R<br />
Ph<br />
• after !-H elimination the olefin is coordinated to Pd-H, so if Pd-H is not<br />
induced to reductively eliminate readdition to the olefin may occur<br />
olefin isomerization in the product<br />
olefin isomerization in the starting material<br />
<strong>Heck</strong> product with the<br />
wrong stereochemistry<br />
• bases help to minimize olefin isomerization by promoting reductive elimination of PdH<br />
R<br />
H L<br />
Pd<br />
X<br />
Ph<br />
Ph<br />
R<br />
H<br />
Pd<br />
X<br />
L<br />
NR 3<br />
X - H + NR 3<br />
L 2 Pd(0)<br />
• halide scavengers help to minimize olefin isomerization by aiding in the reductive elimination of PdH<br />
Ph<br />
R<br />
Pd<br />
L X<br />
reinsert<br />
R<br />
H L<br />
Pd<br />
X<br />
Ph<br />
Ph<br />
R<br />
H<br />
Pd<br />
X<br />
L<br />
AgOAc<br />
Ag-X<br />
L 2 Pd(0)<br />
!-Hydride Elimination - continued<br />
• syn !-H elimination defines the E-selectivity of <strong>Heck</strong> products<br />
R<br />
Ph<br />
Pd<br />
L<br />
X<br />
migratory<br />
insertion<br />
H<br />
H<br />
Ph<br />
Pd<br />
H<br />
R<br />
L X<br />
internal<br />
rotation<br />
Ph<br />
H<br />
H<br />
Pd<br />
R<br />
H<br />
L X<br />
(sterically congested<br />
conformer)<br />
Ph<br />
H<br />
H<br />
Pd<br />
L X<br />
R<br />
H<br />
Pd<br />
L<br />
X<br />
R<br />
H<br />
Ph<br />
• a Z-selective <strong>Heck</strong> reaction can be explained by several factors:<br />
PdH induced isomerization of starting material<br />
PdH induced isomerization of product<br />
different mechanism for !-H elimination (eg. anti !-H elimination)<br />
9