The Chemistry of Dyes Part I: The Synthesis of Indigo Dye
The Chemistry of Dyes Part I: The Synthesis of Indigo Dye
The Chemistry of Dyes Part I: The Synthesis of Indigo Dye
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>The</strong> <strong>Chemistry</strong> <strong>of</strong> <strong><strong>Dye</strong>s</strong><br />
<strong>Part</strong> I: <strong>The</strong> <strong>Synthesis</strong> <strong>of</strong> <strong>Indigo</strong> <strong>Dye</strong><br />
Name:_______________<br />
Period:____<br />
PURPOSE: To synthesize indigo dye in preparation for understanding how and why it dyes fabrics<br />
THEORY: Organic <strong>Chemistry</strong> is the study <strong>of</strong> the structure and reactions <strong>of</strong> the compounds <strong>of</strong> carbon. Many<br />
<strong>of</strong> the most useful compounds produced by the chemical industry are organic. From gasoline to plastic wrap to<br />
the building blocks <strong>of</strong> life, the products <strong>of</strong> organic chemistry can be found everywhere.<br />
One <strong>of</strong> the more interesting areas <strong>of</strong> organic chemistry is the chemistry <strong>of</strong> dyes. <strong><strong>Dye</strong>s</strong> are typically organic<br />
compounds that absorb light in specific areas <strong>of</strong> the visible spectrum. Natural dyes have been used for<br />
thousands <strong>of</strong> years and are typically obtained from various forms <strong>of</strong> plant life. Indeed, indigo is one <strong>of</strong> these<br />
dyes as it can be obtained from various plants. <strong>The</strong> primary plant from which indigo was obtained was called<br />
Indig<strong>of</strong>ere Sumatrana, found primarily in India. Indeed, one <strong>of</strong> the primary reasons for such vigorous trading<br />
between India and the West was due to the high demand for the rich color that indigo could provide to fabrics.<br />
<strong>Indigo</strong> belongs to a class <strong>of</strong> dyes called vat dyes. Vat dyes are perhaps the oldest dyes known, and the<br />
word vat is used to described how the dyes were obtained from plant or other material. After the plant was<br />
cultivated, it was placed in a vessel called a vat to extract the dye. <strong>The</strong> vat was typically filled with water and<br />
fermentation by bacteria within the plant would extract the dye as a soluble solid. <strong>The</strong> vat would then be<br />
agitated (usually be men running in the vat!) causing the dye to precipitate out <strong>of</strong> solution in its characteristic<br />
purple color.<br />
We know that the chemistry <strong>of</strong> a molecule is based on its structure and distribution <strong>of</strong> electrons. It is these<br />
qualities that make indigo an excellent dye. But what are the qualities <strong>of</strong> a good dye? For most people the dye<br />
should produce a desired color, be even, meaning that it colors the material uniformly, and colorfast, meaning<br />
that the dye should not wash out <strong>of</strong> the clothes (and perhaps stain other pieces <strong>of</strong> clothing in the wash). We can<br />
apply our knowledge <strong>of</strong> chemistry to the first question now, and we will be able to answer the other two<br />
questions by the time we actually dye a material with the indigo.<br />
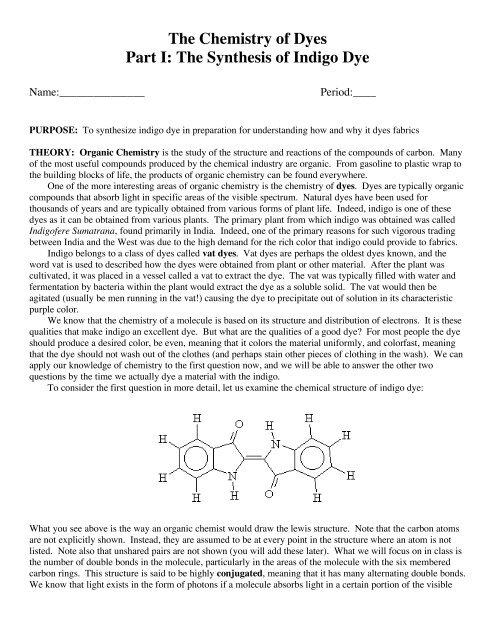
To consider the first question in more detail, let us examine the chemical structure <strong>of</strong> indigo dye:<br />
What you see above is the way an organic chemist would draw the lewis structure. Note that the carbon atoms<br />
are not explicitly shown. Instead, they are assumed to be at every point in the structure where an atom is not<br />
listed. Note also that unshared pairs are not shown (you will add these later). What we will focus on in class is<br />
the number <strong>of</strong> double bonds in the molecule, particularly in the areas <strong>of</strong> the molecule with the six membered<br />
carbon rings. This structure is said to be highly conjugated, meaning that it has many alternating double bonds.<br />
We know that light exists in the form <strong>of</strong> photons if a molecule absorbs light in a certain portion <strong>of</strong> the visible
spectrum. <strong>The</strong> large number <strong>of</strong> alternating double bonds allows the electrons in the molecule to absorb light in<br />
the visible spectrum. Most dyes will have this highly conjugated structure.<br />
Today we will synthesize indigo dye. Unfortunately the actual reaction is fairly complicated, and we will<br />
not delve into the actual steps <strong>of</strong> the process. However, the overall reaction can be presented as follows:<br />
<strong>The</strong> sodium hydroxide added acts as a catalyst for the reaction. After you collect the product, you will do some<br />
simple stoichiometry to determine the limiting reactant and the percent yield <strong>of</strong> the reaction. One interesting<br />
thing to think about: the product you make is insoluble in water. How then does it dye jeans? This is the<br />
question we will answer in part II <strong>of</strong> this lab later this year.<br />
MATERIALS:<br />
Large Vial Organic Reaction Vial<br />
Hot water setup with immersion heater<br />
Ice water beaker<br />
Two beral pipets<br />
Copper Wire<br />
10 ml graduated cylinder<br />
Buchner Funnel and vacuum filtration setup<br />
CHEMICALS<br />
o-nitrobenzaldehyde<br />
acetone<br />
sodium hydroxide<br />
Data Table I – Masses <strong>of</strong> Reactants and Products<br />
Mass <strong>of</strong> o-nitrobenzaldehyde (g)<br />
Volume <strong>of</strong> acetone added (ml)<br />
Volume <strong>of</strong> sodium hydroxide added (ml)<br />
Mass <strong>of</strong> Weighing boat<br />
Mass <strong>of</strong> Weighing Boat + <strong>Indigo</strong> <strong>Dye</strong><br />
Cost <strong>of</strong> 10.0 g <strong>of</strong> o-nitrobenzaldehyde<br />
OBSERVATIONS AFTER ACETONE IS ADDED TO SOLUTION:
OBSERVATIONS AFTER SODIUM HYDROXIDE IS ADDED TO SOLUTION:<br />
OBSERVATIONS AFTER FILTERING AND WEIGHING:<br />
CALCULATIONS:<br />
1) Based on your pre-lab assignment the night before, rewrite the structural equation given above as a<br />
standard balanced chemical equation showing the actual molecular formulas <strong>of</strong> each reactant and<br />
product<br />
2) <strong>The</strong> first thing we need to establish is what is the limiting reactant in this reaction. To do this we need<br />
the number <strong>of</strong> moles <strong>of</strong> each reactant. First calculate the molar masses <strong>of</strong> acetone and o-<br />
nitrobenzaldehyde.<br />
Molar Mass <strong>of</strong> Acetone:<br />
Molar Mass <strong>of</strong> o-nitrobenzaldehyde:<br />
3) We now need the mass <strong>of</strong> acetone. Discover a way to calculate the mass from your data table and a<br />
suitable literature reference. Explain how you calculated the mass (a calculation will suffice) and list<br />
where you obtained your information to help you calculate this mass:
4) Now calculate the limiting reactant in this reaction. Clearly show all work:<br />
5) Finally, calculate the theoretical and percent yield <strong>of</strong> your sample <strong>of</strong> indigo. Again, clearly show all<br />
work:<br />
6) Based on your percent yield, how many grams o-nitrobenzaldehyde would you need to start with if you<br />
wanted to produce 1.0 pound <strong>of</strong> indigo? Explain with calculations<br />
7) Your data table has the cost <strong>of</strong> 10.0 g <strong>of</strong> o-nitrobenzaldehyde. Based on this and your answer to 6,<br />
would it be cheaper for you to make 1.0 pound <strong>of</strong> indigo or buy it from a supplier? (Of course you will<br />
need to find a supplier <strong>of</strong> indigo on the web. Please include a website I can look up to verify the<br />
supplier price)<br />
CONCLUSION:
PROCEDURE: <strong>The</strong> synthesis <strong>of</strong> indigo<br />
Day One<br />
1) Form a lab group <strong>of</strong> two people and put on aprons, goggles, and rubber gloves!<br />
2) Add approximately 350 ml <strong>of</strong> distilled water to a 400 ml beaker. Place an immersion heater in the<br />
water, plug it in, and allow the water to come to a boil. DO NOT PULG IN THE HEATER UNTIL<br />
IT HAS BEEN IMMERSED IN THE WATER.<br />
3) At your station you will filled a preweighed bottle vial <strong>of</strong> o-nitrobenzaldehyde. Record the mass <strong>of</strong> the<br />
reactant in the data table and remove the label from the vial.<br />
4) Have a member <strong>of</strong> your group go to the reagent station with your 10 ml graduated cylinder and obtain<br />
2.5 ml <strong>of</strong> acetone. Record the volume <strong>of</strong> the acetone in the graduated cylinder and add the acetone to the<br />
reaction vial. Gently mix the solution to dissolve the o-nitrobenzaldehyde.<br />
5) Rinse the graduated cylinder with distilled water and then return to the reagent station to obtain 4 ml <strong>of</strong><br />
sodium hydroxide. Add the sodium hydoxide to your reaction vial and quickly seal the vial with the<br />
Teflon cap. Make sure the cap is tight and shake the reaction mixture again.<br />
6) Wrap a small piece (approx. 6 inches) <strong>of</strong> copper wire around the cap to serve as a handle.<br />
7) Place the sealed vial in the beaker containing the boiling water. If at any time, small bubbles start<br />
coming out <strong>of</strong> the vial, the cap is not on tight enough. Remove the vial, allow it to cool, and retighten<br />
the cap.<br />
8) After 3-4 minutes, remove the vial from the hot water using the copper handle and place it on the<br />
tabletop. Allow the mixture to cool for 3-4 minutes or until it is cool enough to touch. Vigorously<br />
shake the vial. Tighten the cap again and place the vial back in the boiling water.<br />
9) While the solution is boiling, prepare a Buchner funnel for vacuum filtration by adding a piece <strong>of</strong> filter<br />
paper to the funnel and gently bathe the filter paper with water. In addition, create an ice water bath<br />
using a Styr<strong>of</strong>oam cup filled with an ice / distilled water mixture<br />
10) After 10-12 minutes, use the copper wire handle to remove the vial from the boiling water bath and<br />
place on the counter for a few minutes to cool.<br />
11) Place the vial in the cold water bath you created for a few minutes.<br />
12) Carefully open the reaction vial. <strong>The</strong> contents <strong>of</strong> the vial are likely pressurized and some solution may<br />
squirt out. This will not be a problem if you open the vial slowly.<br />
13) Complete the final setup <strong>of</strong> your Buchner funnel (Ask if you need help) and gently pour your mixture<br />
into the middle <strong>of</strong> the funnel. Rinse the vial with distilled water twice to make sure you have removed<br />
all product. <strong>The</strong>n wash the product twice with distilled water from your ice bath.<br />
14) Allow the vacuum filtration setup to run for 2-3 minutes. <strong>The</strong>n remove the filter paper containing your<br />
product and place on a labeled watch glass to dry at least overnight.<br />
Day Two:<br />
15) Find the mass <strong>of</strong> an empty weighing boat<br />
16) After the indigo is dry, transfer the product to a massed weighing boat. Obtain the mass <strong>of</strong> the product<br />
and boat and place this mass in the data table.<br />
17) Place your group’s indigo in the provided film canister. Label and give to the instructor to save for part<br />
II <strong>of</strong> the lab later in the semester.