You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
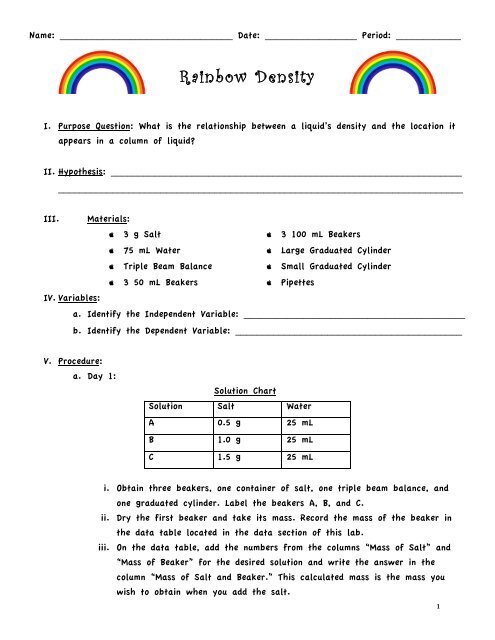
Name: ________________________________ Date: _________________ Period: ____________<br />
<strong>Rainbow</strong> <strong>Density</strong><br />
I. Purpose Question: What is the relationship between a liquid’s density and the location it<br />
appears in a column of liquid?<br />
II. Hypothesis: _________________________________________________________________<br />
___________________________________________________________________________<br />
III. Materials:<br />
apple 3 g Salt apple 3 100 mL Beakers<br />
apple 75 mL Water apple Large Graduated Cylinder<br />
apple Triple Beam Balance apple Small Graduated Cylinder<br />
apple 3 50 mL Beakers apple Pipettes<br />
IV. Variables:<br />
a. Identify the Independent Variable: _________________________________________<br />
b. Identify the Dependent Variable: __________________________________________<br />
V. Procedure:<br />
a. Day 1:<br />
Solution Chart<br />
Solution Salt Water<br />
A 0.5 g 25 mL<br />
B 1.0 g 25 mL<br />
C 1.5 g 25 mL<br />
i. Obtain three beakers, one container of salt, one triple beam balance, and<br />
one graduated cylinder. <strong>Lab</strong>el the beakers A, B, and C.<br />
ii. Dry the first beaker and take its mass. Record the mass of the beaker in<br />
the data table located in the data section of this lab.<br />
iii. On the data table, add the numbers from the columns “Mass of Salt” and<br />
“Mass of Beaker” for the desired solution and write the answer in the<br />
column “Mass of Salt and Beaker.” This calculated mass is the mass you<br />
wish to obtain when you add the salt.<br />
1
iv. Add the amount of salt specified in the Solution Chart to the beaker.<br />
Adjust the triple beam balance until the desired mass is obtained (This is<br />
the mass calculated in step ii).<br />
v. Use water from your sink and obtain 25 mL of water, measured in the<br />
graduated cylinder.<br />
vi. Pour the 25 mL of water into the beaker that has the salt in it. Stir the<br />
solution until the salt is completely dissolved.<br />
vii. Mass the beaker with the salty water in it. Record the mass in the data<br />
table.<br />
viii. Repeat steps ii-v for Solution B and Solution C, using the different beaker<br />
each time.<br />
VI. Discussion Questions:<br />
a. Day 1:<br />
i. How do the volumes of the solutions compare to one another? Does adding<br />
different amounts of salt increase the volume at different levels? ___________<br />
___________________________________________________________________<br />
___________________________________________________________________<br />
ii. How do the masses of the solutions compare to one another? ________________<br />
___________________________________________________________________<br />
___________________________________________________________________<br />
iii. Calculate the density of each solution. Find the mass of the solution from the<br />
Data Table, in the column labeled “Mass of Salt and Water.” Find the volume<br />
of the solution from the “Solution Chart” on page 1.<br />
Solution <strong>Density</strong> (<strong>Density</strong>= Mass/Volume)<br />
A<br />
B<br />
C<br />
iv. Predict what you think will happen if the solutions were put into a test tube.<br />
If Solution A was red, Solution B was yellow, and Solution C was blue, draw<br />
what you think the test tube would look like. ____________________________<br />
___________________________________________________________________<br />
2
VII.<br />
________________________________________________________<br />
Procedure<br />
a. Day 2:<br />
i. Obtain 10 mL of Solutions A, B, and C. Measure out the 10 mL in the<br />
smallest graduated cylinder then three small beakers.<br />
ii. Pipette 2 full pipettes of each liquid into one test tube as instructed by<br />
your teacher at the beginning of the period. Do this in the following<br />
order: Blue, Yellow, and then Red.<br />
iii. Make a drawing of the test tube once you have completed your pipetting (do<br />
this in the space provided in the data section of this lab packet).<br />
VIII.<br />
Data:<br />
a. Data Table:<br />
Solution Mass of Salt<br />
Mass of<br />
Beaker<br />
Mass of Beaker<br />
and Salt<br />
Mass of Beaker and<br />
Salt and Water<br />
Mass of Salt<br />
and Water<br />
A<br />
0.5 g<br />
B<br />
1.0 g<br />
C<br />
1.5 g<br />
b. Test Tube Drawings<br />
Solution A (Red) Solution B (Yellow) Solution C (Blue)<br />
3
IX. Discussion Questions:<br />
a. Day 2:<br />
i. Did your result turn out as you expected? (See Day 1 Question 4) Explain<br />
your answer. _____________________________________________________<br />
________________________________________________________________<br />
________________________________________________________________<br />
ii. List the layers, by color, in the order of least to most dense.<br />
1.<br />
2.<br />
3.<br />
iii. If the volume of each layer is the same, explain why their densities are<br />
different. ________________________________________________________<br />
________________________________________________________________<br />
________________________________________________________________<br />
iv. Predict what you think would happen if you inverted the test tube. Then,<br />
invert the test tube. Explain what you observe and why it happened. _____<br />
________________________________________________________________<br />
________________________________________________________________<br />
X. Conclusion: (State if your hypothesis was right, why or why not? Use data to support<br />
your answer. Be sure to answer the purpose question and make a short summary<br />
statement about the results of the lab.)<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
___________________________________________________________________________<br />
4