You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
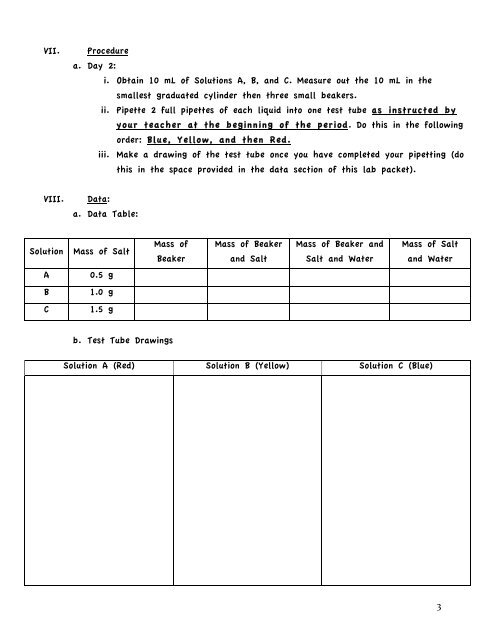
VII.<br />
________________________________________________________<br />
Procedure<br />
a. Day 2:<br />
i. Obtain 10 mL of Solutions A, B, and C. Measure out the 10 mL in the<br />
smallest graduated cylinder then three small beakers.<br />
ii. Pipette 2 full pipettes of each liquid into one test tube as instructed by<br />
your teacher at the beginning of the period. Do this in the following<br />
order: Blue, Yellow, and then Red.<br />
iii. Make a drawing of the test tube once you have completed your pipetting (do<br />
this in the space provided in the data section of this lab packet).<br />
VIII.<br />
Data:<br />
a. Data Table:<br />
Solution Mass of Salt<br />
Mass of<br />
Beaker<br />
Mass of Beaker<br />
and Salt<br />
Mass of Beaker and<br />
Salt and Water<br />
Mass of Salt<br />
and Water<br />
A<br />
0.5 g<br />
B<br />
1.0 g<br />
C<br />
1.5 g<br />
b. Test Tube Drawings<br />
Solution A (Red) Solution B (Yellow) Solution C (Blue)<br />
3