van't Hoff factor
van't Hoff factor
van't Hoff factor
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
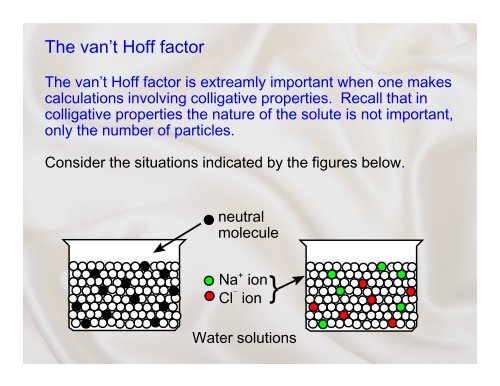
The van’t <strong>Hoff</strong> <strong>factor</strong><br />
The van’t <strong>Hoff</strong> <strong>factor</strong> is extreamly important when one makes<br />
calculations involving colligative properties. Recall that in<br />
colligative properties the nature of the solute is not important,<br />
only the number of particles.<br />
Consider the situations indicated by the figures below.<br />
neutral<br />
molecule<br />
Na + ion<br />
Cl G ion<br />
}<br />
Water solutions