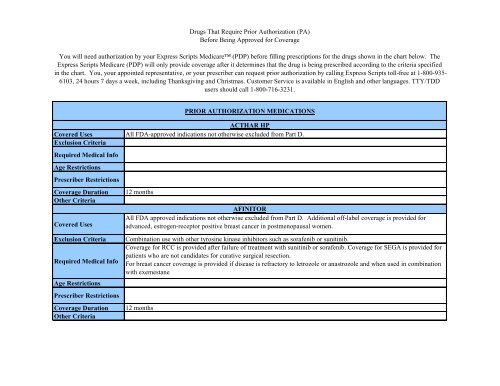
Drugs That Require Prior Authorization (PA) Before ... - Express Scripts
Drugs That Require Prior Authorization (PA) Before ... - Express Scripts
Drugs That Require Prior Authorization (PA) Before ... - Express Scripts
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCOMETRIQAll FDA approved indications not otherwise excluded from Part D. Plus patients already started on Cometriq for aCovered Use.Diagnosis of progressive, metastatic medullary thyroid cancer.<strong>Authorization</strong> will be for 12 months.CRINONEAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label use is provided tosupport established pregnancyCoverage is not provided for infertility9 months for support of established pregnancy, 12 months for secondary amenorrhea
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoDARBEPOETIN ALFAAll FDA-approved indications not otherwise excluded from Part D. Additional off-label coverage is provided for anemiasecondary to myelodysplasia.Treatment of anemia due to CRF for patients on dialysis is provided when either 1. Hgb/Hct is less than 30% or 10g/dLOR 2. patient is symptomatic or has required a transfusion. Treatment of anemia due to CRF/CRI for patients NOT ondialysis is provided when either 1. Hgb/Hct is less than 30% or 10g/dL OR 2. patient is symptomatic or has required atransfusion. Anemia due to cancer-chemotherapy is provided when 1. patient is currently receiving myelosuppressivechemotherapy or it has been 6 weeks or less following the completion of the final dose of myelosuppressivechemotherapy AND 2. Hgb/Hct is less than 30% or 10 g/dL. Myelodysplasia related anemia is provided when 1.Hgb/Hct is less than 30% or 10 g/dL AND 2. erythropoietin level is less than or equal to 500 units/L.Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria3 months - anemia due to cancer-chemotherapy, 12 months - CRF/CRI or myelodysplasia related anemiaRenewals coverage for anemia due to CRF for patients on dialysis is provided when either 1. Hgb/Hct is less than 33%or 11g/dL OR 2. prescriber indicates the dose will be held or titrated downward. Renewals coverage for anemia due toCRF/CRI for patients NOT on dialysis is provided when either 1. Hgb/Hct is less than 30% or 10g/dL OR 2. prescriberindicates the dose will be held or titrated downward. Renewals for myelodysplasia related anemia is provided when 1.Hgb/Hct is less than or equal to 36% or 12 g/dL AND 2. in the presence of therapeutic benefit, if Hgb/HCT hasincreased or stabilized, or if the need for transfusions has decreased. Patients renewing for cancer-chemotherapy relatedanemia must meet required medical information.
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoEPOETIN ALFAAll FDA-approved indications not otherwise excluded from Part D. Additional off-label coverage is provided for anemiasecondary to HIV infection or HIV drug therapy, myelodysplasia, and chronic hepatitis C treatment from ribavirin andinterferon therapy.Treatment of anemia due to CRF for patients on dialysis is provided when either 1. Hgb/Hct is less than 30% or 10g/dLOR 2. patient is symptomatic or has required a transfusion. Treatment of anemia due to CRF/CRI for patients NOT ondialysis is provided when either 1. Hgb/Hct is less than 30% or 10g/dL OR 2. patient is symptomatic or has required atransfusion. Anemia due to cancer-chemotherapy is provided when 1. patient is currently receiving myelosuppressivechemotherapy or it has been 6 weeks or less following the completion of the final dose of myelosuppressivechemotherapy AND 2. Hgb/Hct is less than 30% or 10 g/dL. Myelodysplasia related anemia is provided when 1.Hgb/Hct is less than 30% or 10 g/dL AND 2. erythropoietin level is less than or equal to 500 units/L. Treatment ofanemia due to HIV infection or HIV drug therapy is provided when 1. Hgb/Hct is less than 33% or 11 g/dL OR 2.erythropoietin is less than or equal to 500 units/L AND patient is symptomatic or has required transfusions. Anemia dueto chronic hepatitis C treatment from ribavirin and interferon therapy is provided when Hgb/Hct is less than or equal to33% or 11 g/dL. For use in reducing the need for allogenic blood transfusions in surgery patients is provided when 1.the surgery is elective, non-vascular or non-cardiac AND 2. Hgb is less than or equal to 13 g/dL AND 3. patient refusesor cannot undergo autologous donation prior to surgery.Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria1 month- allogeneic blood transfusions, 3 months - chemotherapy, 12 months - other indicationsRenewals coverage for anemia due to CRF for patients on dialysis is provided when either 1. Hgb/Hct is less than 33%or 11g/dL OR 2. prescriber indicates the dose will be held or titrated downward. Renewals coverage for anemia due toCRF/CRI for patients NOT on dialysis is provided when either 1. Hgb/Hct is less than 30% or 10g/dL OR 2. prescriberindicates the dose will be held or titrated downward. Renewals for myelodysplasia related anemia is provided when 1.Hgb/Hct is less than or equal to 36% or 12 g/dL AND 2. in the presence of therapeutic benefit, if Hgb/HCT hasincreased or stabilized, or if the need for transfusions has decreased. Renewals for anemia due to HIV infection or HIVdrug therapy is provided when Hgb/Hct is less than or equal to 36% or 12 g/dL. Patients renewing anemia due to cancerchemotherapyor chronic hepatitis C treatment from ribavirin and interferon therapy must meet required medicalinformation.
ERIVEDGECovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion CriteriaFor locally advanced BCC, the patient must have disease recurrence following surgery and radiation or not be a<strong>Require</strong>d Medical Info candidate for surgery and radiation.Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical Info12 monthsFENTANYL TRANSMUCOSALAll FDA-approved indications not otherwise excluded from Part DCoverage is not provided for concurrent use with other transmucosal fentanyl productsCoverage is provided for use in the management of breakthrough CANCER pain that cannot be managed successfullywith oral immediate-release narcotics and where the patient is already tolerant to long-acting narcotic analgesicsAge RestrictionsPrescriber RestrictionsCoverage Duration 12 monthsOther CriteriaGILOTRIFCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion CriteriaFor NSCLC - EGFR exon deletions or mutations<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria<strong>Authorization</strong> will be for 12 months.For the treatment of metastatic non small cell lung cancer (NSCLC) must be used in tumors with epidermal growthfactor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoGROWTH HORMONESAll FDA approved indications not otherwise excluded from Part D. Additional off-label coverage is provided for (note -some growth hormone drugs may be labeled for 1 or more of these indications): adult growth hormone deficiency,growth failure in children small for gestational age or with intrauterine growth retardation, idiopathic short stature, GHdeficiency associated with Turner Syndrome, growth failure secondary to chronic renal failure/insufficiency in childrenwho have not received a renal transplant, short stature associated with Noonan Syndrome, and for the treatment ofPrader-Willi Syndrome.Coverage is not provided for constitutional delayed growthPediatric GHD: epiphyses must be confirmed open in patients 10 years of age and older, AND 1. diagnosis confirmedby any 2 provocative tests or by both low IGF-1 and IGFBP-3 levels in patients who meet the height related conditionsof coverage, 2. diagnosis confirmed by 2 provocative tests and both low IGF-1 and IGF-BP3 in patients not meetingheight related coverage conditions, or 3. 3 pituitary hormone deficiencies in pt with irreversible hypothalamic-pituitarystructural lesions or panhypopituitarism. Growth failure from CRF: PGHD criteria must be met without the provocativetests or IGF-1 and IGF-BP3 related conditions. Idiopathic Short Stature: epiphyses must be confirmed as open inpatients greater than or equal 10 years of age, height must be less than or equal - 2.25 sds from the mean. Small forGestational Age: failure to manifest catch up growth by age 2 defined as birth weight, birth length, or both that are morethan 2 sds mean normal values following adjustment for age and gender. Turner’s syndrome and Noonan Syndrome:epiphyses must be confirmed as open and when on therapy. Adult GHD: requires either 1. a negative GH provocativetest when the AGHD is due to childhood onset GHD, pituitary or hypothalamic disease, surgery or radiation therapy, ortrauma, OR 2. 3 pituitary hormone deficiencies and baseline serum IGF-I levels below the age- and sex-appropriatereference range when the AGHD is due to irreversible hypothalamic-pituitary structural lesions or panhypopituitarismnot acquired as a child, OR 3. 3 pituitary hormone deficiencies if adult panhypopit or irreversible hypothalamicpituitarystructural lesions are from childhood. Short bowel syndrome: when receiving specialized nutritional support.
Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaPediatric endocrinologist for ISS1 month for short bowel syndrome, 12 months for other indicationsHeight related conditions of coverage – 1. height below the third percentile for their age and gender related height, 2.growth velocity subnormal greater than or equal 2 standard deviations (sds) from the age related mean, 3. delayedskeletal maturation greater than or equal 2 sds below the age/gender related mean. Renewals for PGHD, CFR, SGA,Turner's and Noonan Syndromes require growth response of greater than or equal 4.5 cm/yr (pre-pubertal) or greaterthan or equal 2.5 cm/yr (post-pubertal) AND open epiphyses. For pediatric patients with irreversible hypothalamicpituitarystructural lesions or panhypopituitarism coverage is renewable if the patient has had 3 pituitary hormonedeficiencies. Renewals for short bowel syndrome is provided in the presence of clinical benefit (such as, decreasing thepatient’s intravenous nutritional requirements). Renewals for Prader-Willi syndrome is provided if pt has increase inlean body mass or decrease in fat mass. Renewals for ISS is provided in the presence of a growth response of greaterthan or equal 1.5 cm/yr AND open epiphyses. Renewals for AGHD is provided in the presence of clinical benefit (e.g.,increase in total lean body mass, increase in IGF-1 and IGFBP-3 levels, or increase in exercise capacity).Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoHUMIRAAll FDA approved indications not otherwise excluded from Part D. Additional off-label coverage is provided formoderate to severe ulcerative colitisCoverage is not provided for use of Humira in combination with other biologics e.g., Enbrel, Kineret or Remicade, etcCoverage is provided in situations where the patient has been evaluated and screened for the presence of latent TBinfection, where warranted, prior to initiating treatment with Humira.Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 monthsRenewal coverage is provided in situations where treatment has provided clinical benefit.
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoICLUSIGAll FDA approved indications not otherwise excluded from Part D. Plus patients already started on Iclusig for a CoveredUse.Diagnosis for which Iclusig is being used. For chronic myelogenous leukemia (CML), prior therapies tried must bereported to confirm resistance or intolerance to prior tyrosine kinase inhibitor therapy. For acute lymphoblastic leukemia(ALL), the Philadelphia chromosome (Ph) status of the leukemia must be reported. For ALL, prior therapies must bereported to document resistance or intolerance to prior tyrosine kinase inhibitor therapy.Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical Info<strong>Authorization</strong> will be for 12 months.For CML, patient must have resistance or intolerance to prior tyrosine kinase inhibitor therapy for approval. For ALL,patient must have Ph-positive ALL and must have resistance or intolerance to prior tyrosine kinase inhibitor therapy forapproval.IMMUNE GLOBULINS - INTRAVENOUSAll FDA-approved indications not otherwise excluded from Part D. Additional off-label coverage is provided for postbone marrow transplantation, autoimmune hemolytic anemia, Guillain-Barre syndrome, chronic inflammatorydemyelinating polyneuropathy, corticosteroid treatment resistant dermatomyositis, multifocal neuropathy, myastheniagravis, and pediatric HIV infection.For Primary immune deficiency and B cell CLL - patient must have history of a recurrent bacterial infection or a singlelife-threatening bacterial infection AND IgG levels less than or equal to 600 mg/dL. For ITP - platelets must be lessthan or equal to 30,000/mm3. For bone marrow transplantation (BMT) - BMT must be performed within the previous100 days AND patient is not also receiving CMV immune globulinAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoINCIVEK - HEP C - PROTEASE INHIBITORSAll FDA-approved indications not otherwise excluded from Part DCoverage is not provided for genotypes other than type 1. Previous failure to Incivek or Victrelis.Chronic Hep C, in patients with genotype 1 who have a quantifiable viral load. Must be used in combination with apegylated interferon and ribavirin.Age RestrictionsPrescriber RestrictionsCoverage Duration 3 monthsOther CriteriaINCRELEXCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion CriteriaFor severe primary IGFD coverage is provided if patient’s height standard deviation score must be less than or equal -3.0 AND the basal IGF-1 score must be below the lower limits of normal for the reporting lab AND the patient musthave normal or elevated growth hormone AND epiphyses must be confirmed as open in patients greater than or equal 10<strong>Require</strong>d Medical Infoyears of age. For GH gene deletion coverage is provided if presence of neutralizing antibodies to growth hormones andopen epiphyses in patients older than 10 years old. A growth response of greater than or equal 4.5 cm/yr (pre-pubertalgrowth phase) or greater than or equal 2.5 cm/yr (post-pubertal) must occur for continuation of coverage.Age RestrictionsPrescriber RestrictionsCoverage is provided in situations where the diagnosis of IGF-1 deficiency has been made by an endocrinologist.Coverage Duration 12 monthsOther CriteriaINLYTACovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria Combination use with other tyrosine kinase inhibitors such as sorafenib, sunitinibFor RCC coverage is provided after failure with one prior systemic therapy<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
JAKAFICovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage Duration 12 monthsOther CriteriaKALYDECOCovered Uses All FDA-approved indications not otherwise excluded from Part D.Coverage is NOT provided in patients who are homozygous for F508 deletion or who have other mutations in the CFTRExclusion Criteria gene.<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCoverage is provided in the treatment of cystic fibrosis in patients who have a G551D mutation in the CFTR gene asdetected by a FDA cleared CF mutation test.12 monthsCoverage may be renewed in situations where the patient is continuing to derive benefit from treatment (for example,improved or stable lung function measured by FEV1)LETAIRISCovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion CriteriaCoverage is provided for use in combination with two or more <strong>PA</strong>H therapies when treatment with one <strong>PA</strong>H agent<strong>Require</strong>d Medical Infofailed to adequately control the patient’s symptoms.Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCoverage is provided in situations where it is being prescribed under the care or referral of a cardiologist orpulmonologist.12 monthsRenewal coverage is provided in situations where treatment has provided clinical benefit.
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaLIDODERMAll FDA-approved indications not otherwise excluded from Part D. Additional off-label coverage is provided fordiabetic neuropathy.Coverage is not provided for neuropathic pain other than postherpetic neuralgia or diabetic peripheral neuropathy (forexample, radiculopathy, or chemotherapy related neuropathy).For the off-label use of diabetic neuropathy: the patient must have previous use and inadequate response or intoleranceto any ONE neuropathic pain medication, including (but not limited to): tri-cyclic antidepressants, gabapentin, Lyrica,opioids, venlafaxine, Cymbalta.12 monthsMEKINISTAll FDA-approved indications not otherwise excluded from Part D. Plus patients already started on Mekinist for aCovered Use.Diagnosis for which Mekinist is being used. For unresectable or metastatic melanoma must have documentation ofBRAF V600E or V600K mutations<strong>Authorization</strong> will be for 12 months.For unresectable or metastatic melanoma must be used in patients with BRAF V600E or V600K mutations
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoMULTIPLE SCLEROSIS THERAPYAll FDA-approved indications not otherwise excluded from Part D. Additional off-label coverage is provided fortreatment at the time of a first demyelinating event.Treatment of primary progressive MS is not covered. Combination therapy with a beta interferon product, Gilenya, orCopaxone is not covered.For relapsing forms of multiple sclerosis: Patient must still either be able to walk at least a few steps or alternativelymust have some functional arm/ hand use consistent with performing activities of daily living. For Rebif only, patientsmust be already receiving Rebif or have experienced intolerance/failure with glatiramer (Copaxone), interferon beta-1b(Betaseron) or Interferon beta-1a (Avonex).Age RestrictionsPrescriber RestrictionsCoverage Duration 12 monthsOther CriteriaMULTIPLE SCLEROSIS THERAPY- GILENYACovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsTreatment of primary progressive MS is not covered. Combination therapy with a beta interferon product or Copaxoneis not covered.Patient must still either be able to walk at least a few steps or alternatively must have some functional arm/ hand useconsistent with performing activities of daily living.Coverage Duration 12 monthsOther CriteriaNEUMEGACovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsPatient has experienced severe thrombocytopenia (e.g., platelet count less than equal to 20,000/mcL) from previouschemotherapy OR for patient is considered to be at high risk for the development of severe thrombocytopenia.Coverage DurationOther Criteria12 months
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoNEXAVARAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label uses in thetreatment of gastrointestinal stromal tumors, metastatic thyroid cancer, relapsed/refractory or metastatic osteosarcoma,angiosarcoma, and advanced/unresectable desmoid tumors is provided.Combination use with other tyrosine kinase inhibitors such as sorafenib, sunitinibFor GIST - coverage is provided after disease progression with imatinib and sunitinib. For Osteosarcoma, patients musthave tried standard chemotherapy.Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 monthsORENCIA - SUBCUTANEOUSAll FDA-approved indications not otherwise excluded from Part DCoverage is not provided for use in combination with other biologics e.g., Humira, Kineret, Remicade, etcCoverage is provided in situations where the patient has been evaluated and screened for the presence of latent TBinfection, where warranted, prior to initiating treatment. Coverage is provided in situations where the patientexperienced intolerance/failure to Humira AND Enbrel.18 years of age or older5 yearsRenewal coverage is provided in situations where treatment has provided clinical benefit.OXANDROLONEAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label use is providedfor cachexia associated with AIDSFor weight gain - must be used to promote weight gain after weight loss resulting from chronic diseases or AIDSwasting, per section 1927(k)(6) of the Act5 years
<strong>PA</strong>GET'S DISEASE AGENTSCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria Treatment of osteoporosis<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber Restrictions12 monthsPERJETAAll FDA approved indications not otherwise excluded from Part D. Coverage is provided for the off-label use of iHER2positive metastatic breast cancer in patients with disease progression following treatment with trastuzumab.Patients must have HER2 overexpression of advanced or metastatic breast cancer AND either 1. have not receivedprevious anti-HER2 therapy or chemotherapy and pertuzumab will be used in combination with trastuzumab anddocetaxel OR 2. had disease progression following treatment with trastuzumab and pertuzumab will be used incombination with trastuzumab.Coverage Duration LifetimeOther CriteriaPROMACTACovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion Criteria Coverage is not provided when used in combination with Nplate.For ITP - Patients must have had an inadequate response, intolerance to, or not be a candidate for treatment with<strong>Require</strong>d Medical Infocorticosteroids, immunoglobulins, or splenectomy. For HepC thrombocytopenia - the degree of thrombocytopeniawould not allow the initiation and maintenance of interferon-based therapy.Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 monthsRenewal is provided for patients who continue to have a response to therapy (for example, platelet count has increased)
PROVIGILCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion CriteriaFor narcolepsy, the prescriber must confirm that the patient does not have underlying conditions that may contribute toexcessive sleepiness (e.g., nocturnal myoclonus, current drug therapy which affects sleep or contributes to daytimesedation, or chronic voluntary or involuntary sleep deprivation through shift-work) OR any underlying conditions havebeen addressed and treated. Coverage is provided for SWSD when: (1) Prescriber must confirm that the patient is anight worker and (2) has complaints of persistent and frequent excessive sleepiness and/or falling asleep while at work<strong>Require</strong>d Medical Infoand (3) any medical conditions known to cause or contribute to sleepiness have been considered and treated. Coverageis provided for idiopathic hypersomnolence that is confirmed by polysomnography where excessive sleepiness is notdue to other sleep disorders such as narcolepsy, obstructive sleep apnea or posttraumatic hypersomnia.Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical Info12 monthsFor OSAHS, coverage is provided for patients who are receiving nasal continuous positive airway pressure therapy(C<strong>PA</strong>P) or who are not candidates for C<strong>PA</strong>P. Coverage is provided for depression associated with fatigue and/orsleepiness when the patient is receiving antidepressant therapyREGRANEXAll FDA-approved indications not otherwise excluded from Part D. Coverage is provided for the following off-labeluse: treatment of severe pressure ulcers that are unresponsive to other measures.Treatment of venous status ulcersRegranex must be used as an adjunct to good ulcer wound care (e.g., debridement, infection control and/or pressurerelief).Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria5 monthsFor the off-label use- treatment of severe pressure ulcers ONLY: prescriber must indicate that wound has beenunresponsive to at least ONE other measure (may include but is not limited to the following: nutritionalsupplementation, pressure relief, debridement, proteolytic enzymes, epidermal growth factor).
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaREMICADEAll FDA-approved indications not otherwise excluded from Part D. Additional coverage for off-label use is providedfor moderate to severe Hidradenitis suppurativa, Behcet's disease, Still's disease, uveitis, sarcoidosis, pyodermagangrenosum, graft vs host disease, and juvenile idiopathic arthritis.Coverage is not provided for use of Remicade in combination with other biologics e.g., Enbrel, Kineret or Humira.Coverage is provided in situations where the patient has been evaluated and screened for the presence of latent TBinfection prior to initiating treatment with Remicade.Prescribed by or in consultation with: Still’s disease/JIA-rheumatologist, Pyoderma gangrenosum-dermatologist, Uveitisophthalmologist,GVHD-a physician affiliated with a transplant center, oncologist, or hematologist,Behcet’s Diseaserheumatologist,dermatologist, ophthalmologist, gastroenterologist, or neurologist, Sarcoidosis-pulmonologist,ophthalmologist, or dermatologist.12 monthsCoverage is provided for mild ulcerative colitis when the patient has had an inadequate response to at least oneconventional treatment (for example: sulfasalazine, olsalazine, mesalamine, etc.). Coverage is provided for Hidradenitissuppurativa when the patient has tried one other tx (eg, intralesional/oral CSs, systemic antibiotic, isotretinoin. ForBehcet's disease pt must try at least one conventional therapy (e.g. systemic CSs, immunosuppressants (e.g., AZA,MTX, MM, CSA, tacrolimus, chlorambucil, cyclophosphamide) or interferon alfa), Enbrel or Humira. For Still'sdisease - must try a corticosteroid and one non-biologic DMARD (eg, MTX) for at least 2 mos, or was intolerant to anon-biologic DMARD. For Uveitis - Tried periocular/intraocular CSs, systemic CSs, or immunosuppressants (e.g.,MTX, MM, CSA, AZA, CPM), Enbrel, or Humira. Sarcoidosis- Tried CS and immunosuppressive agent (e.g., MTX,AZA, CSA, chlorambucil), or chloroquine, or thalidomide. Pyoderma ganrenosum -Tried one systemic CS or 1immunosuppressant (eg, mycophenolate, CSA), for 2 mos. GVHD.Tried one conventional txment (eg, high-dose CSs,antithymocyte globulin, CSA, thalidomide, tacrolimus, MM, etc.) or receiving Remicade concurrently. JIA approve ifRemicade started in combination with MTX or one other traditional DMARD (eg, leflunomide, sulfasalazine) AND thept has tried 1 other agent for this condition (eg, MTX, sulfasalazine, or leflunomide, an NSAID, or one biologicDMARD [eg, Humira, Orencia, Enbrel, Kineret, Actemra]) or the pt has aggressive disease.
REMODULINCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCoverage is provided in situations where it is being prescribed under the care or referral of a cardiologist orpulmonologist.12 monthsCoverage is provided for use in combination with two or more <strong>PA</strong>H therapies when treatment with one <strong>PA</strong>H agentfailed to adequately control the patient’s symptoms.REVATIOCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsCoverage is provided in situations where it is being prescribed under the care or referral of a cardiologist orPrescriber Restrictionspulmonologist.Coverage Duration 12 monthsCoverage is provided for use in combination with two or more <strong>PA</strong>H therapies when treatment with one <strong>PA</strong>H agentOther Criteriafailed to adequately control the patient’s symptoms.RIBAVIRINCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
Covered UsesExclusion CriteriaRITUXANAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label use is providedfor relapsed or refractory Waldenstrom’s macroglobulinemia.Coverage is not provided for use of Rituxan in combination with other biologics e.g., Humira, Kineret or Remicade, etc.<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaFor rheumatoid arthritis: 18 years of age or older1 month for rheumatoid arthritis, 12 months for other indicationsFor rheumatoid arthritis: must be used in combination with MTX and after inadequate response or intolerance to at leastone TNF inhibitor.Coverage is provided for Antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (e.g., Wegener’sGranulomatosis (WG) or Microscopic Polyangiitis (M<strong>PA</strong>)) when used in combination with glucocorticoids.SIGNIFORCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion CriteriaDiagnosis for which Signifor is being used.<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCushing's - 18 years of age and olderInitial course - Prescribed by or in consultation with an endocrinologist3 months for initial therapy and 12 months for continuation therapyFor Cushing's disease, Signifor will be approved if according to the prescribing physician the patient is not a candidatefor surgery or surgery has not been curative. Patients who have already been started on Signifor for Cushing's diseasewill be approved if the patient has had a response, as determined by the prescribing physician and patient is continuingtherapy to maintain response.
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoSIMPONIAll FDA-approved indications not otherwise excluded from Part DCoverage is not provided for use in combination with other biologics e.g., Humira, Kineret, Remicade, etcCoverage is provided in situations where the patient has been evaluated and screened for the presence of latent TBinfection, where warranted, prior to initiating treatment with Simponi. For RA or AS - Coverage is provided insituations where the patient experienced intolerance/failure to Humira AND Enbrel. For UC - Coverage is provided insituations where the pt experienced intolerance/failure to Humira. For rheumatoid arthritis, Simponi must be used incombination with methotrexate, per labeling.Age RestrictionsPrescriber RestrictionsCoverage Duration 12 monthsOther Criteria Renewal coverage is provided in situations where treatment has provided clinical benefit.SIMPONI ARIACovered Uses All FDA-approved indications not otherwise excluded from Part D.Concurrent use with another biologic (for example, tocilizumab, certolizumab, etanercept, adalimumab, anakinra,Exclusion Criteria abatacept, infliximab, rituximab) or tofacitinib.<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaDiagnosis for which Simponi Aria is being prescribed, concurrent medications, previous therapies tried.RA - adultsRA - Prescribed by or in consultation with a rheumatologist<strong>Authorization</strong> will be for 12 months.For RA - in patients who have not been receiving Simponi or Simponi Aria, approve if they will be taking Simponi Ariain combination with MTX or one other traditional disease-modifying antirheumatic drug (DMARD) [e.g., leflunomide,sulfasalazine, hydroxychloroquine], unless intolerant or contraindicated AND meets one of the following criteria: i.Patient has tried one DMARD (brand or generic, oral or injectable) for at least 3 months, (this includes patients whohave tried other biologic DMARDs for at least 3 months) OR ii. Patient has a contraindication or intolerance to MTXand leflunomide, as determined by the prescribing physician OR iii. Patient has early RA (defined as disease duration ofless than 6 months) with at least one of the following features of poor prognosis - functional limitation (e.g., based onhealth assessment questionnaire disability index (HAQ-DI) score), extraarticular disease such as rheumatoid nodules,RA vasculitis, or Felty’s syndrome, positive rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibodies, orbony erosions by radiograph.
SMOKING DETERRENTSCovered Uses All FDA-approved indications not otherwise excluded from Part D.Combination use of more than one nicotine replacement product. Bupropion sustained-release tablet (Zyban) used inExclusion Criteria combination with another form of bupropion.<strong>Require</strong>d Medical InfoThe patient must be enrolled in a behavioral support/ modification program (e.g., community program, manufacturersponsored program, counseling by the physician, internet, or telephone quitline).Age RestrictionsPrescriber RestrictionsThe patient must be 18 years of age or olderCoverage Duration 12 monthsOther CriteriaSOMAVERTCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion CriteriaCoverage is provided when patients have had an inadequate response to surgery, radiation, or other medical therapies or<strong>Require</strong>d Medical Info in situations where the patient is not a candidate for other therapies.Age RestrictionsPrescriber RestrictionsCoverage Duration 12 monthsOther CriteriaSTIVARGACovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion CriteriaFor metastatic colorectal cancer, as per labeling coverage, is provided when the patient has been previously treated withfluoropyrimidine-, oxaliplatin-, and irinotecan- based chemotherapy AND anti-VEGF therapy (for example,bevacizumab, etc), AND for known KRAS wild type tumors, when the patient was previously treated with an anti-<strong>Require</strong>d Medical InfoEGFR therapy (for example, panitumumab, cetuximab, etc). Coverage is NOT provided for use in combination withother kinase inhibitors (for example sorafenib, etc.) For gastrointestinal stromal tumors (GIST), as per labeling,authorization for Stivarga may be given in patients who have been previously treated with imatinib and sunitinib.Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
Covered UsesSUTENTAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label use is providedfor metastatic thyroid cancer, recurrent chordoma, angiosarcoma, solitary fibrous tumor/hemangiopericytoma, andalveolar soft part sarcoma.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCombination use with other kinase inhibitors (for example, sorafenib, etc).Coverage is provided for Gastrointestinal stromal tumor when the patient had evidence of disease progression orexperienced intolerance while receiving imatinib mesylate (Gleevec).Coverage Duration 12 monthsOther CriteriaSYLATRONCovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion CriteriaAdjunctive treatment of Stage III resected melanoma<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage Duration 12 monthsOther CriteriaSYNRIBOCovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion CriteriaFor chronic phase or accelerated phase CML pts must have resistance or intolerance to prior therapy with at least TWO<strong>Require</strong>d Medical Infotyrosine kinase inhibitors (for example, dasatinib, nilotinib, imatinib, bosutinib, etc) and Synribo must NOT be used incombination with other kinase inhibitors (for example sorafenib, sunitinib, etc.)Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria18 years of age or older12 months
Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoTAFINLARAll FDA-approved indications not otherwise excluded from Part D. Plus patients already started on Tafinlar for aCovered Use.Diagnosis for which Tafinlar is being used. For unresectable or metastatic melanoma must have documentation ofBRAF V600E mutation<strong>Authorization</strong> will be for 12 months.For unresectable or metastatic melanoma must be used in patients with BRAF V600E mutationTARCEVAAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label use is providedfor newly diagnosed glioblastoma multiformeCoverage is provided for treatment of pancreatic cancer when used in combination with gemcitabine.Coverage is provided for newly diagnosed glioblastoma multiforme when used in combination with temozolomideduring and after radiotherapy.Age RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
TECFIDERACovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion CriteriaThe patient must have a relapsing form of MS, (relapsing forms of MS include relapsing-remitting multiple sclerosis[RRMS], secondary-progressive multiple sclerosis [SPMS] with relapses, and progressive-relapsing multiple sclerosis[PRMS]) and Tecfidera must not be used concurrently with other disease-modifying agents used for MS (i.e., Avonex,Rebif, Betaseron, Extavia, Copaxone, Tysabri, Gilenya, and Aubagio). Patients who have not yet received Tecfideramust also meet one of the following conditions: 1. be unable to administer injections due to dexterity issues or visual<strong>Require</strong>d Medical Infoimpairment, 2. have tried two of the following: Avonex, Rebif, Betaseron/Extavia, or Copaxone, 3. the patient has triedCopaxone but cannot take an interferon beta therapy (i.e., Avonex, Rebif, and Betaseron/Extavia) due to any of thefollowing A. having depression, being suicidal, or having a severe psychiatric disorder B. being a woman who ispregnant or plans to become pregnant C. having active liver disease or a history of significant liver disease or D.having a history of seizures.Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesPrescribed by, or in consultation with, a neurologist or a physician who specializes in the treatment of MS12 monthsTHALIDOMIDEAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label uses includeCrohn's disease, aphthous ulcers in the presence of HIV or AIDS, prostate cancer, malignant melanoma, myelofibrosis,myelodysplastic syndromes, and advanced hepatocellular carcinoma.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
TORISELCovered Uses All FDA approved indications not otherwise excluded from Part D.Coverage is not provided when used in combination with interferon alfa or kinase inhibitors (for example, sorafenib,Exclusion Criteria sunitinib, etc).<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage Duration 12 monthsOther CriteriaTRACLEERCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage is provided in situations where it is being prescribed under the care or referral of a cardiologist orpulmonologist.Coverage DurationOther Criteria12 monthsCoverage is provided for use in combination with two or more <strong>PA</strong>H therapies when treatment with one <strong>PA</strong>H agentfailed to adequately control the patient’s symptoms.VACCINES HPVCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsCoverage for Gardasil provided for patients 9 through 26 years of age. Coverage for Cervarix is provided for femalesbetween 9 and 25 years of age.Prescriber RestrictionsCoverage DurationOther Criteria12 months
VACCINES SHINGLESCovered Uses All FDA-approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoCoverage is provided for patients 50 years of age and older.12 monthsVICTRELIS- HEP C - PROTEASE INHIBITORSAll FDA-approved indications not otherwise excluded from Part DCoverage is not provided for genotypes other than type 1. Previous failure to Incivek or Victrelis.Chronic Hep C, in patients with genotype 1 who have a quantifiable viral load. Must be used in combination with apegylated interferon and ribavirin.Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical Info11 monthsXALKORIAll FDA approved indications not otherwise excluded from Part D. Additional coverage for off-label use is providedfor inflammatory myofibroblastic tumor (IMT) with ALK translocationALK positive measured by either Abbott's Vysis ALK Break Apart FISH probe test or another reliable CLIA approvedtesting method (for example RT-PCR, FISH, or IHC) locally advanced or metastatic NSCLCAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
Covered UsesExclusion CriteriaXELJANZAll FDA approved indications not otherwise excluded from Part D. Plus patients already started on Xeljanz for aCovered Use.Concurrent use with a biologic for an inflammatory condition (eg, tocilizumab, anakinra, abatacept, rituximab) or a TNFinhibitor (eg, certolizumab pegol, etanercept, adalimumab, infliximab, golimumab).Concurrent use with potent immunosuppressants that are not methotrexate (MTX) [eg, azathioprine, tacrolimus,cyclosporine, mycophenolate mofetil].<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaRheumatoid Arthritis (RA), adults.RA, prescribed by or in consultation with a rheumatologist.<strong>Authorization</strong> will be for 12 months.Approve in RA patients who have had a trial with at least two of the following - tocilizumab, anakinra, abatacept,rituximab, certolizumab, etanercept, adalimumab, infliximab, golimumab. Approve in RA patients who have tried oneof the following- etanercept (Enbrel) OR adalimumab (Humira) or Orencia SC for at least 3 months unless intolerant.Covered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaXGEVAAll FDA-approved indications not otherwise excluded from Part DCoverage is not provided for prevention of skeletal-related events in patients with multiple myelomaPrevention of skeletal-related events in patients with bone metastases from solid tumors12 months
XOLAIRCovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion CriteriaBaseline IgE must be greater than or equal to 30 IU/mL. The patient is currently receiving therapy with an inhaledsteroid or oral steroid unless the patient should not receive steroids AND either 1. The dose of inhaled or systemicsteroid must be reduced to help control adverse side effects and addition of Xolair is the only option that may achieve<strong>Require</strong>d Medical Info the needed dosage reduction OR 2. The patient has moderate to severe asthma defined as having had two or more ERvisits for an asthma exacerbation AND/OR more than 2 courses of short pulse oral or parenteral corticosteroids forexacerbations within the previous 12 monthsAge RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCoverage is provided for patients 12 years of age and older12 monthsCoverage may be renewed in situations where treatment if providing clinical benefit as evidenced by a reduction inasthma exacerbations from baseline.XTANDICovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage is provided for the treat of metastatic castration-resistant prostate cancer in situations where the patient hasbeen previously treated with a docetaxel based therapy.Coverage Duration 12 monthsOther CriteriaXYREMCovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 months
YERVOYCovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber Restrictions18 years of age or olderCoverage Duration 4 monthsOther CriteriaZALTRAPCovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion CriteriaCoverage is provided for the treatment of metastatic colorectal cancer in situations where the patient had evidence of<strong>Require</strong>d Medical Info disease progression or resistance to an oxaliplatin containing regimen AND when used in combination with 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI).Age RestrictionsPrescriber RestrictionsCoverage DurationOther CriteriaCovered UsesExclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber Restrictions12 monthsZELBORAFAll FDA-approved indications not otherwise excluded from Part D. Additional coverage for off-label use includesunresectable or metastatic melanoma in patients with BRAFv600K mutationCombination use with ipilimumabFor unresectable or metastatic melanoma with BRAFV600E or BRAFV600K mutation as detected by FDA approved orCLIA lab approved reliable assayCoverage DurationOther Criteria12 months
ZYTIGACovered Uses All FDA approved indications not otherwise excluded from Part D.Exclusion Criteria<strong>Require</strong>d Medical InfoAge RestrictionsPrescriber RestrictionsCoverage DurationOther Criteria12 monthsCoverage is provided in situations where the patient will be using this drug in combination with oral prednisone.The following drugs may be covered under Medicare Part B or D depending upon the circumstances. Information may need to besubmitted describing the use and setting of the drug to make the determination.Drug NameACETYLCYSTEINERoute of Administration / Dosage FormINHALATION SOLUTIONALBUTEROL SULFATE INHALATION NEBULIZATION SOLUTIONAMPHOTERICIN BAZATHIOPRINEBUDESONIDECALCITRIOLCALCITRIOLCALCITRIOLCELLCEPTINJECTION SOLUTION RECONSTITUTEDORAL TABLETINHALATION SUSPENSIONORAL CAPSULEINJECTION SOLUTIONORAL SOLUTIONORAL SUSPENSION RECONSTITUTEDCROMOLYN SODIUM INHALATION NEBULIZATION SOLUTIONCUBICINCYCLOPHOSPHAMIDE ORAL TABLETCYCLOSPORINECYCLOSPORINEMODIFIEDINJECTION SOLUTION RECONSTITUTEDORAL CAPSULEORAL CAPSULE
Drug NameCYCLOSPORINEMODIFIEDDRONABINOLEMENDENGERIX-BRoute of Administration / Dosage FormORAL SOLUTIONORAL CAPSULEORAL CAPSULEINJECTION SUSPENSIONFOSCARNET SODIUM INJECTION SOLUTIONGENGRAFORAL SOLUTIONGENGRAFORAL CAPSULEGRANISETRON HCL ORAL TABLETIPRATROPIUMBROMIDEINHALATION SOLUTIONIPRATROPIUMBROMIDE/ALBUTERO INHALATION SOLUTIONL SULFATELEVALBUTEROL INHALATION NEBULIZATION SOLUTIONLEVALBUTEROL HCL INHALATION NEBULIZATION SOLUTIONLEVOCARNITINE ORAL TABLETLEVOCARNITINE ORAL SOLUTIONLIORESALINTRATHECALINJECTION SOLUTIONMETHOTREXATE ORAL TABLETMETHYLPREDNISOLOORAL TABLETNEMYCOPHENOLATEMOFETILORAL TABLETMYCOPHENOLATEMOFETILORAL CAPSULEMYFORTICORAL TABLET DELAYED RELEASENEBUPENTINHALATION SOLUTION RECONSTITUTEDNEORALORAL CAPSULE
Drug NameNEORALNITROGLYCERINONDANSETRON HCLONDANSETRON HCLONDANSETRON ODTPERFOROMISTPREDNISOLONESODIUM PHOSPHATEPREDNISONEPREDNISONEPREDNISONEINTENSOLPULMICORTPULMOZYMERA<strong>PA</strong>MUNERA<strong>PA</strong>MUNERECOMBIVAX HBSANDIMMUNESANDIMMUNETACROLIMUSTOBITYVASOVANCOMYCIN HCLZEMPLARZEMPLARZORTRESSS5660_OT39111IS5983_OT39111IRoute of Administration / Dosage FormORAL SOLUTIONINJECTION SOLUTIONORAL SOLUTIONORAL TABLETORAL TABLET DISPERSIBLEINHALATION NEBULIZATION SOLUTIONORAL SOLUTIONORAL SOLUTIONORAL TABLETORAL CONCENTRATEINHALATION SUSPENSIONINHALATION SOLUTIONORAL SOLUTIONORAL TABLETINJECTION SUSPENSIONORAL CAPSULEORAL SOLUTIONORAL CAPSULEINHALATION NEBULIZATION SOLUTIONINHALATION SOLUTIONINJECTION SOLUTION RECONSTITUTEDORAL CAPSULEINJECTION SOLUTIONORAL TABLETCMS Approval Date 01/13/2010No changes made since 11/2013